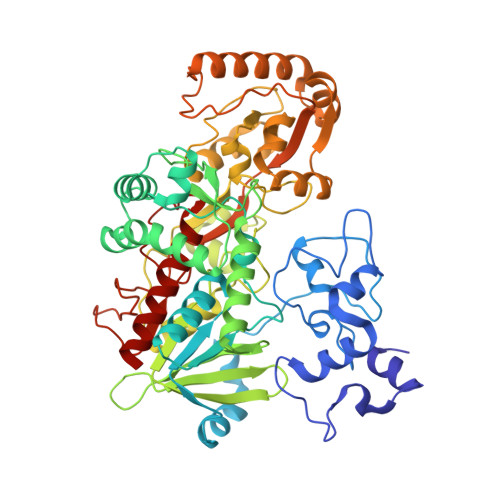
Structural and Mechanistic Mapping of a Unique Fumarate Reductase
Taylor, P., Pealing, S.L., Reid, G.A., Chapman, S.K., Walkinshaw, M.D.(1999) Nat Struct Biol 6: 1108
- PubMed: 10581550
- DOI: https://doi.org/10.1038/70045
- Primary Citation of Related Structures:
1QJD - PubMed Abstract:
The 1.8 A resolution crystal structure of the tetraheme flavocytochrome c3, Fcc3, provides the first mechanistic insight into respiratory fumarate reductases or succinate dehydrogenases. The multi-redox center, three-domain protein shows a 40 A long 'molecular wire' allowing rapid conduction of electrons through a new type of cytochrome domain onto the active site flavin, driving the reduction of fumarate to succinate. In this structure a malate-like molecule is trapped in the enzyme active site. The interactions between this molecule and the enzyme suggest a clear mechanism for fumarate reduction in which the substrate is polarized and twisted, facilitating hydride transfer from the reduced flavin and subsequent proton transfer. The enzyme active site in the oxidized form is completely buried at the interface between the flavin-binding and the clamp domains. Movement of the cytochrome and clamp domains is postulated to allow release of the product.
- Institute of Cell and Molecular Biology, University of Edinburgh, Mayfield Road, Edinburgh EH9 3JR, UK.
Organizational Affiliation: