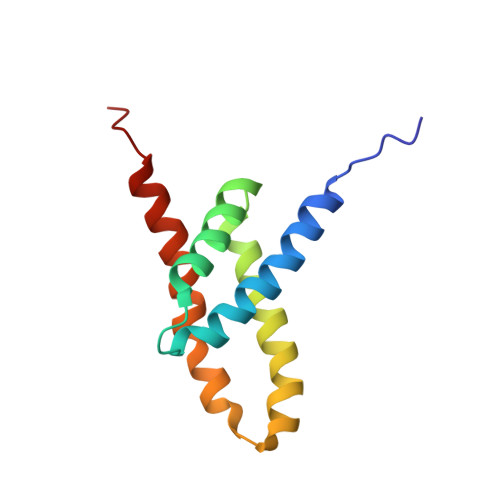
NMR structure of the KaiC-interacting C-terminal domain of KaiA, a circadian clock protein: implications for KaiA-KaiC interaction
Vakonakis, I., Sun, J., Wu, T., Holzenburg, A., Golden, S.S., LiWang, A.C.(2004) Proc Natl Acad Sci U S A 101: 1479-1484
- PubMed: 14749515
- DOI: https://doi.org/10.1073/pnas.0305516101
- Primary Citation of Related Structures:
1Q6A, 1Q6B - PubMed Abstract:
KaiA is a two-domain circadian clock protein in cyanobacteria, acting as the positive element in a feedback loop that sustains the oscillation. The structure of the N-terminal domain of KaiA is that of a pseudo-receiver, similar to those of bacterial response regulators, which likely interacts with components of the clock-resetting pathway. The C-terminal domain of KaiA is highly conserved among cyanobacteria and enhances the autokinase activity of KaiC. Here we present the NMR structure of the C-terminal domain of KaiA from the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. This domain adopts a novel all alpha-helical homodimeric structure. Several mutations known to affect the period of the circadian oscillator are shown to be located at an exposed groove near the dimer interface. This NMR structure and a 21-A-resolution electron microscopy structure of the hexameric KaiC particle allow us to postulate a mode of KaiA-KaiC interaction, in which KaiA binds a linker region connecting two globular KaiC domains.
- Departments of Biochemistry and Biophysics, Texas A&M University, College Station, TX 77843, USA.
Organizational Affiliation: