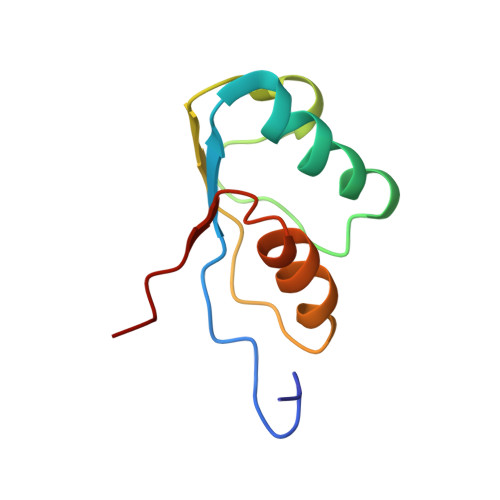
The NMR structure of the activation domain isolated from porcine procarboxypeptidase B.
Vendrell, J., Billeter, M., Wider, G., Aviles, F.X., Wuthrich, K.(1991) EMBO J 10: 11-15
- PubMed: 1989879
- DOI: https://doi.org/10.1002/j.1460-2075.1991.tb07915.x
- Primary Citation of Related Structures:
1PBA - PubMed Abstract:
The three-dimensional structure of the activation domain isolated from porcine pancreatic procarboxypeptidase B was determined using 1H NMR spectroscopy. A group of 20 conformers is used to describe the solution structure of this 81 residue polypeptide chain, which has a well-defined backbone fold from residues 11-76 with an average root mean square distance for the backbone atoms of 1.0 +/- 0.1 A relative to the mean of the 20 conformers. The molecular architecture contains a four-stranded beta-sheet with the polypeptide segments 11-17, 36-39, 50-56 and 75-76, two well defined alpha-helices from residues 20-30 and 60-70, and a 3(10) helix from residues 43-46. The three helices are oriented almost exactly antiparallel to each other, are all on the same side of the beta-sheet, and the helix axes from an angle of approximately 45 degrees relative to the direction of the beta-strands. Three segments linking beta-strands and helical secondary structures, with residues 32-35, 39-43 and 56-61, are significantly less well ordered than the rest of the molecule. In the three-dimensional structure two of these loops (residues 32-35 and 56-61) are located close to each other near the protein surface, forming a continuous region of increased mobility, and the third disordered loop is separated from this region only by the peripheral beta-strand 36-39 and precedes the short 3(10) helix.
- Institut für Molekularbiologie und Biophysik, Eidgenössische, Technische Hochschule--Hönggerberg, Zürich, Switzerland.
Organizational Affiliation: