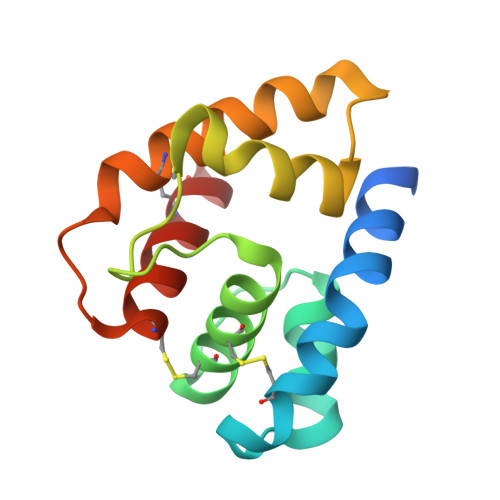
THE CRYSTAL STRUCTURE OF A COCKROACH PHEROMONE-BINDING PROTEIN SUGGESTS A NEW LIGAND BINDING AND RELEASE MECHANISM
Lartigue, A., Gruez, A., Spinelli, S., Riviere, S., Brossut, R., Tegoni, M., Cambillau, C.(2003) J Biological Chem 278: 30213-30218
- PubMed: 12766173
- DOI: https://doi.org/10.1074/jbc.M304688200
- Primary Citation of Related Structures:
1ORG, 1OW4, 1P28 - PubMed Abstract:
Pheromone-binding proteins (PBPs) are small helical proteins found in sensorial organs, particularly in the antennae, of moth and other insect species. They were proposed to solubilize and carry the hydrophobic pheromonal compounds through the antennal lymph to receptors, participating thus in the peri-receptor events of signal transduction. The x-ray structure of Bombyx mori PBP (BmorPBP), from male antennae, revealed a six-helix fold forming a cavity that contains the pheromone bombykol. We have identified a PBP (LmaPBP) from the cockroach Leucophaea maderae in the antennae of the females, the gender attracted by pheromones in this species. Here we report the crystal structure of LmaPBP alone or in complex with a fluorescent reporter (amino-naphthalen sulfonate, ANS) or with a component of the pheromonal blend, 3-hydroxy-butan-2-one. Both compounds bind in the internal cavity of LmaPBP, which is more hydrophilic than BmorPBP cavity. LmaPBP structure ends just after the sixth helix (helix F). BmorPBP structure extends beyond the sixth helix with a stretch of residues elongated at neutral pH and folding as a seventh internalized helix at low pH. These differences between LmaPBP and BmorPBP structures suggest that different binding and release mechanism may be adapted to the hydrophilicity or hydrophobicity of the pheromonal ligand.
- Architecture et Fonction des Macromolécules Biologiques, UMR 6098 CNRS and Universités Aix-Marseille I & 2, 31 Ch. Joseph Aiguier, 13402 Marseille Cedex 20, France.
Organizational Affiliation: