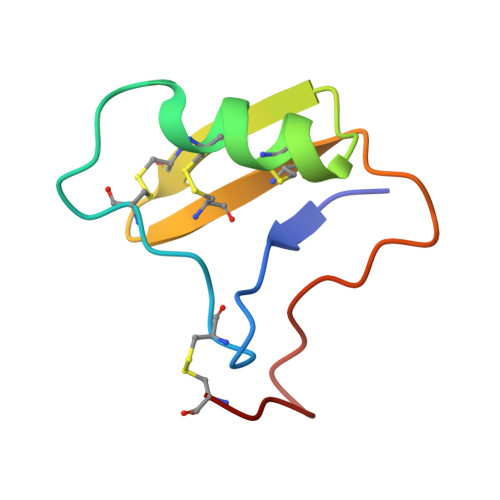
Structural analysis of Tityus serrulatus Ts1 neurotoxin at atomic resolution: insights into interactions with Na+ channels.
Pinheiro, C.B., Marangoni, S., Toyama, M.H., Polikarpov, I.(2003) Acta Crystallogr D Biol Crystallogr 59: 405-415
- PubMed: 12595696
- DOI: https://doi.org/10.1107/s090744490202111x
- Primary Citation of Related Structures:
1NPI - PubMed Abstract:
The structure of the Ts1 toxin from the Brazilian scorpion Tityus serrulatus was investigated at atomic resolution using X-ray crystallography. Several positively charged niches exist on the Ts1 molecular surface, two of which were found to coordinate phosphate ions present in the crystallization solution. One phosphate ion is bound to the conserved basic Lys1 residue at the Ts1 N-terminus and to residue Asn49. The second ion was found to be caged by residues Lys12, Trp54 and Arg56. Lys12 and Tyr/Trp54 residues are strictly conserved in all classical scorpion beta-neurotoxins. The cavity formed by these residues may represent a special scaffold required for interaction between beta-neurotoxins and sodium channels. The charge distribution on the Ts1 surface and the results of earlier chemical modification studies and side-directed mutagenesis experiments strongly indicate that the phosphate-ion positions mark plausible binding sites to the Na(+) channel. The existence of two distinct binding sites on the Ts1 molecular surface provides an explanation for the competition between Ts1, depressant (LqhIT2) and excitatory (AaHIT) neurotoxins.
- Laboratório Nacional de Luz Síncrotron (LNLS), CP 6192, CEP 13084-971, Campinas/SP, Brazil.
Organizational Affiliation: