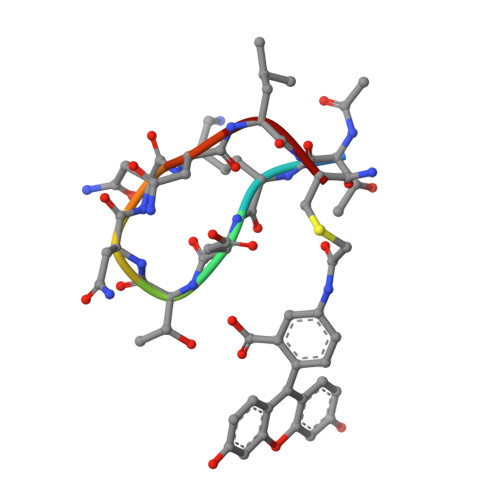
Bactericidal antibody recognition of a PorA epitope of Neisseria meningitidis: crystal structure of a Fab fragment in complex with a fluorescein-conjugated peptide.
van den Elsen, J.M., Herron, J.N., Hoogerhout, P., Poolman, J.T., Boel, E., Logtenberg, T., Wilting, J., Crommelin, D.J., Kroon, J., Gros, P.(1997) Proteins 29: 113-125
- PubMed: 9294871
- DOI: https://doi.org/10.1002/(sici)1097-0134(199709)29:1<113::aid-prot9>3.3.co;2-u
- Primary Citation of Related Structures:
1MPA, 2MPA - PubMed Abstract:
Class 1 outer membrane protein PorA of Neisseria meningitidis is a vaccine candidate against bacterial meningitis. Antibodies against PorA are able to induce complement-mediated bacterial killing and thereby play an important role in protection against meningococcal disease. Bactericidal antibodies are all directed against variable regions VR1 and VR2 of the PorA sequence, corresponding to loops 1 and 4 of a two-dimensional topology model of the porin with eight extracellular loops. We have determined the crystal structure to 2.6 A resolution of the Fab fragment of bactericidal antibody MN12H2 against meningococcal PorA in complex with a linear fluorescein-conjugated peptide TKDTNNNL derived from the VR2 sequence of sero-subtype P1.7,16 (residues 180-187) from meningococcal strain H44/76. The peptide folds deeply into the binding cavity of the Fab molecule in a type I beta-turn, with the minimal P1.16 epitope DTNNN virtually completely buried. The structure reveals H-bonds and van der Waals interactions with all minimal epitope residues and one essential salt bridge between Asp-182 of the peptide and His-31 of the MN12H2 light chain. The key components of the recognition of PorA epitope P1.16 by bactericidal antibody MN12H2 correspond well with available thermodynamic data from binding studies. Furthermore, they indicate the structural basis of an increased endemic incidence of infection by group B meningococci in England and Wales since 1981 associated with the occurrence of an Neisseria meningitidis escape mutant (strain-MC58). The observed three-dimensional conformation of the peptide provides a rationale for the development of a synthetic peptide vaccine against meningococcal disease.
- Department of Pharmaceutics, Faculty of Pharmacy, Utrecht Institute of Pharmaceutical Sciences, Utrecht University, The Netherlands.
Organizational Affiliation: