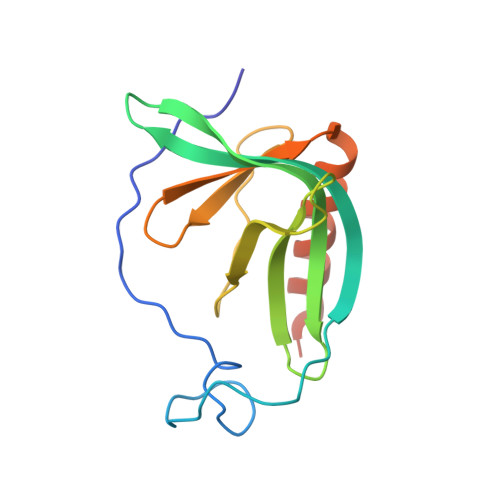
Structure of the N-WASP EVH1 Domain-WIP Complex. Insight into the Molecular Basis of Wiskott-Aldrich Syndrome.
Volkman, B.F., Prehoda, K.E., Scott, J.A., Peterson, F.C., Lim, W.A.(2002) Cell 111: 565-576
- PubMed: 12437929
- DOI: https://doi.org/10.1016/s0092-8674(02)01076-0
- Primary Citation of Related Structures:
1MKE - PubMed Abstract:
Missense mutants that cause the immune disorder Wiskott-Aldrich Syndrome (WAS) map primarily to the Enabled/VASP homology 1 (EVH1) domain of the actin regulatory protein WASP. This domain has been implicated in both peptide and phospholipid binding. We show here that the N-WASP EVH1 domain does not bind phosphatidyl inositol-(4,5)-bisphosphate, as previously reported, but does specifically bind a 25 residue motif from the WASP Interacting Protein (WIP). The NMR structure of the complex reveals a novel recognition mechanism-the WIP ligand, which is far longer than canonical EVH1 ligands, wraps around the domain, contacting a narrow but extended surface. This recognition mechanism provides a basis for understanding the effects of mutations that cause WAS.
- Department of Biochemistry, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Organizational Affiliation: