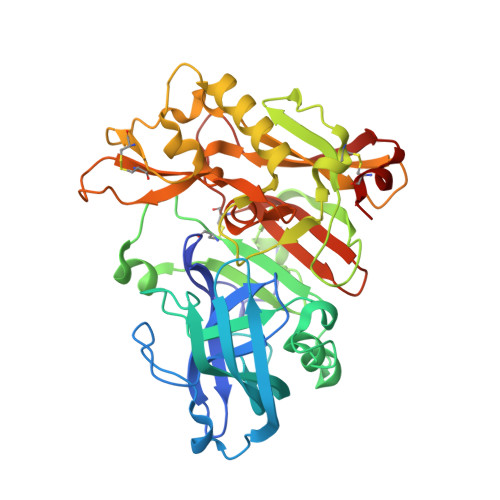
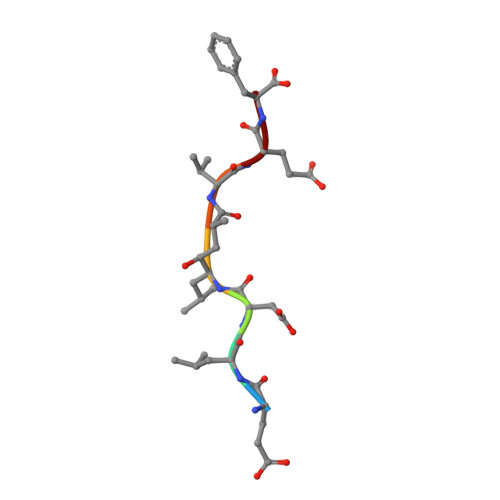
Crystal Structure of Memapsin 2 (beta-Secretase) in Complex with Inhibitor OM00-3
Hong, L., Turner, R.T., Koelsch, G., Shin, D., Ghosh, A.K., Tang, J.(2002) Biochemistry 41: 10963-10967
- PubMed: 12206667
- DOI: https://doi.org/10.1021/bi026232n
- Primary Citation of Related Structures:
1M4H - PubMed Abstract:
The structure of the catalytic domain of human memapsin 2 bound to an inhibitor OM00-3 (Glu-Leu-Asp-LeuAla-Val-Glu-Phe, K(i) = 0.3 nM, the asterisk denotes the hydroxyethylene transition-state isostere) has been determined at 2.1 A resolution. Uniquely defined in the structure are the locations of S(3)' and S(4)' subsites, which were not identified in the previous structure of memapsin 2 in complex with the inhibitor OM99-2 (Glu-Val-Asn-LeuAla-Ala-Glu-Phe, K(i) = 1 nM). Different binding modes for the P(2) and P(4) side chains are also observed. These new structural elements are useful for the design of new inhibitors. The structural and kinetic data indicate that the replacement of the P(2)' alanine in OM99-2 with a valine in OM00-3 stabilizes the binding of P(3)' and P(4)'.
- Protein Studies Program, Oklahoma Medical Research Foundation, and Department of Biochemistry and Molecular Biology, University of Oklahoma Medical Center, Oklahoma City, Oklahoma 73104, USA.
Organizational Affiliation: