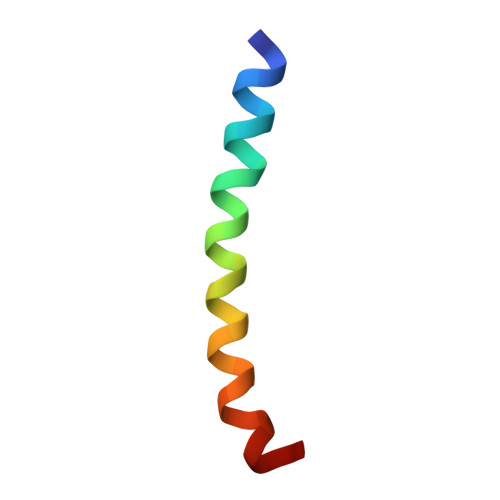
The solution structure of the active domain of CAP18--a lipopolysaccharide binding protein from rabbit leukocytes.
Chen, C., Brock, R., Luh, F., Chou, P.J., Larrick, J.W., Huang, R.F., Huang, T.H.(1995) FEBS Lett 370: 46-52
- PubMed: 7649303
- DOI: https://doi.org/10.1016/0014-5793(95)00792-8
- Primary Citation of Related Structures:
1LYP - PubMed Abstract:
We have employed the circular dichroism (CD) technique to characterize the solution structure of CAP18(106-137), a lipopolysaccharide (LPS) binding, antimicrobial protein, and its interaction with lipid A. Our results revealed that CAP18(106-137) may exist in at least three lipid A concentration-dependent, primarily helix conformations. The 'model' structure of CAP18(106-137) in 30% (v/v) TFE, determined by nuclear magnetic resonance (NMR) technique, was found to be a complete and very rigid helix. In this conformation, the cationic and hydrophobic groups of CAP18(106-137) are separated into patches and stripes in such a way that it can favorably interact with lipid A through either coulombic interaction with the diphosphoryl groups or hydrophobic interaction with the fatty acyl chains.
- Division of Structural Biology, Academia Sinica, Nankang, Taipei, Taiwan, ROC.
Organizational Affiliation: