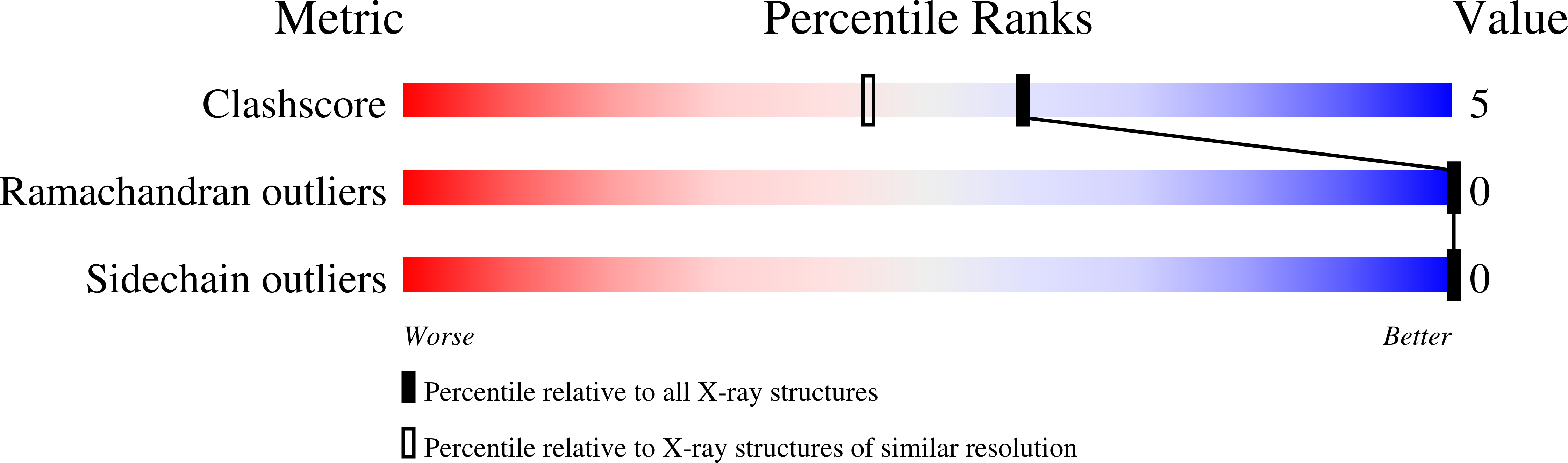Atomic resolution structure of prokaryotic phospholipase A2: Analysis of internal motion and implication for a catalytic mechanism.
Matoba, Y., Sugiyama, M.(2003) Proteins 51: 453-469
- PubMed: 12696056
- DOI: https://doi.org/10.1002/prot.10360
- Primary Citation of Related Structures:
1LWB - PubMed Abstract:
We have found a secreted phospholipase A(2) (PLA(2), EC 3.1.1.4) from Streptomyces violaceoruber A-2688, which is the first PLA(2) identified in prokaryote, and determined its tertiary structure by NMR and X-ray analyses. In this study, we collected the X-ray diffraction data of the bacterial PLA(2) at room temperature (297 K) using conventional MoK(alpha) radiation and refined the structure at a 1.05 A resolution. The atomic resolution analysis led us to introduce disordered conformations and hydrogen atoms into a full anisotropic model. The molecular motion, which is expressed as the sum of rigid-body motion and internal motion of protein, is roughly estimated as the thermal motion when the X-ray diffraction data are collected at room temperature. In this study, we applied a TLS (rigid-body motion in terms of translation, libration, and screw motions) model to analyze the rigid-body motion of the bacterial PLA(2) and calculated the internal motion by subtracting the estimate of the rigid-body motion from the observed anisotropic temperature factor. We also subjected the TLS model to estimate the internal motion of the bovine pancreatic PLA(2) using the anisotropic temperature factor deposited in the Protein Data Bank. Both results indicate that the localization of regions exhibiting larger internal motion in the bacterial PLA(2) is almost the same as that in the bovine pancreatic PLA(2), suggesting that although the tertiary structure of the bacterial PLA(2) is strikingly different from that of the bovine pancreatic PLA(2), the internal motion, which is associated with the calcium(II) ion-binding, phospholipid-binding, and allosteric interfacial activation, is commonly observed in both PLA(2)s.
- Department of Molecular Microbiology and Biotechnology, Graduate School of Biomedical Sciences, Hiroshima University, Hiroshima, Japan.
Organizational Affiliation: