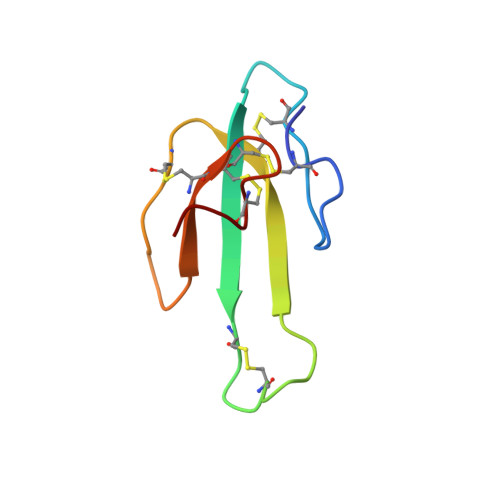
Solution structure of LSIII, a long neurotoxin from the venom of Laticauda semifasciata.
Connolly, P.J., Stern, A.S., Hoch, J.C.(1996) Biochemistry 35: 418-426
- PubMed: 8555211
- DOI: https://doi.org/10.1021/bi9520287
- Primary Citation of Related Structures:
1LSI - PubMed Abstract:
We report the sequence-specific proton assignments and solution structure of the long neurotoxin LSIII from the venom of Laticauda semifasciata determined by two- and three-dimensional 1H NMR. Input for structure calculations consisted of 497 NOE-derived distance restraints and 45 dihedral angle restraints obtained from J couplings. A two-particle-per-residue representation of protein structure was used to generate 200 initial structures which were then subjected to all-atom refinement by simulated annealing. Twenty-three final structures consistent with the experimental restraints were obtained; the average atomic RMS difference between the individual structures and the mean structure was 0.82 A for the backbone heavy atoms and 1.3 A for all heavy atoms (residues 1-26, 37-60). The main elements of regular secondary structure are a three-stranded antiparallel beta-sheet and three finger-like loops protruding from a globular core, consistent with previously reported structures of long neurotoxins. The end of the prominent loop II, which is involved in binding to acetylcholine receptor, is disordered relative to the rest of the molecule. A novel finding of this study is that the loop has a well defined local structure; this and other observations suggest this region moves as a rigid body. We propose that this motion is a heretofore unrecognized general feature of long neurotoxins, with specific consequences for binding to the acetylcholine receptor.
- Rowland Institute for Science, Cambridge, Massachusetts 02142, USA.
Organizational Affiliation: