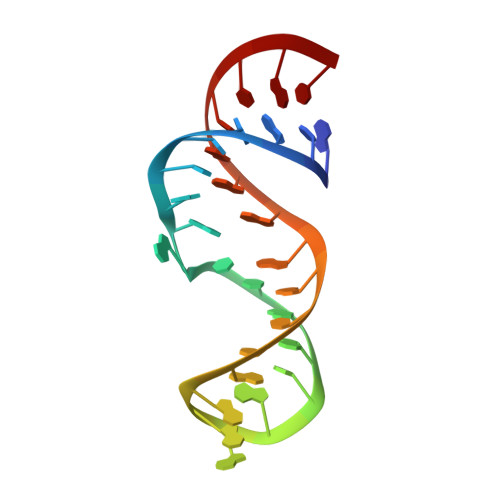
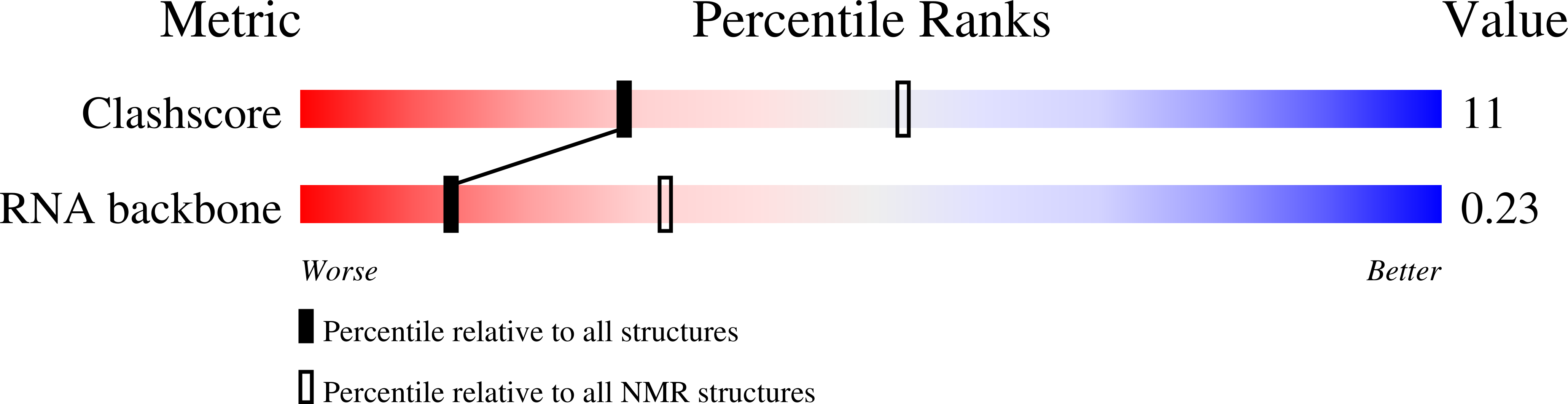NMR solution structure of the lead-dependent ribozyme: evidence for dynamics in RNA catalysis.
Hoogstraten, C.G., Legault, P., Pardi, A.(1998) J Mol Biology 284: 337-350
- PubMed: 9813122
- DOI: https://doi.org/10.1006/jmbi.1998.2182
- Primary Citation of Related Structures:
1LDZ, 2LDZ - PubMed Abstract:
The NMR solution structure of a lead-dependent ribozyme, known as the leadzyme, is presented. This ribozyme is among the smallest of the known catalytic RNAs, with an active site consisting of a six-nucleotide asymmetric internal loop. This loop has a roughly double-helical structure, including a protonated adenine-cytosine wobble base-pair, that positions the cytosine base 5' to the cleavage site in a double-helical conformation. The deviations from helical structure consist of two bulged guanosine residues, G7 and G9, where G7 is the residue 3' to the cleavage site. The scissile phosphate group of the leadzyme is not positioned for in-line nucleophilic attack. Therefore, a conformational rearrangement in the active site is required to reach the proposed transition state for this ribozyme. This is similar to previous observations in X-ray studies of the hammerhead ribozyme, and emphasizes the necessity for dynamic structural fluctuations in the catalytic mechanism of small ribozymes. A model for metal-binding in the leadzyme is proposed in which a lead ion binds to a bulged guanine base that is critical for leadzyme function.
- Department of Chemistry and Biochemistry, University of Colorado Boulder, Campus Box 215, Boulder, CO, 80309, USA.
Organizational Affiliation: