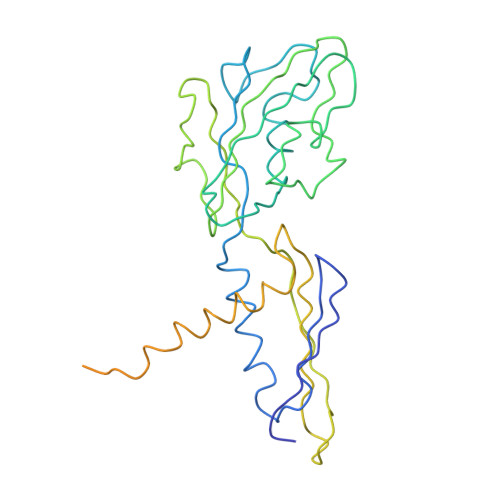
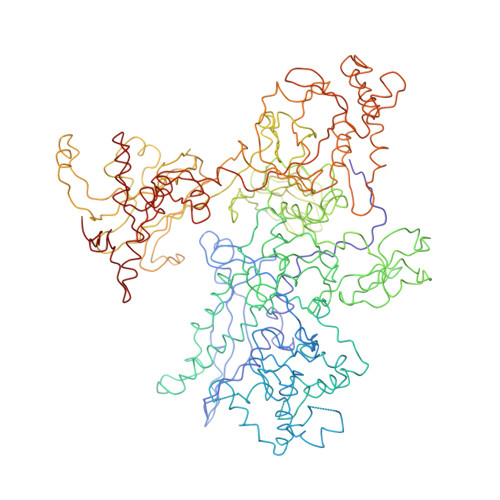
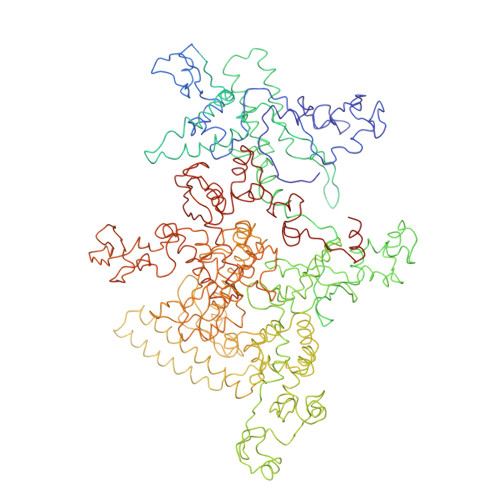
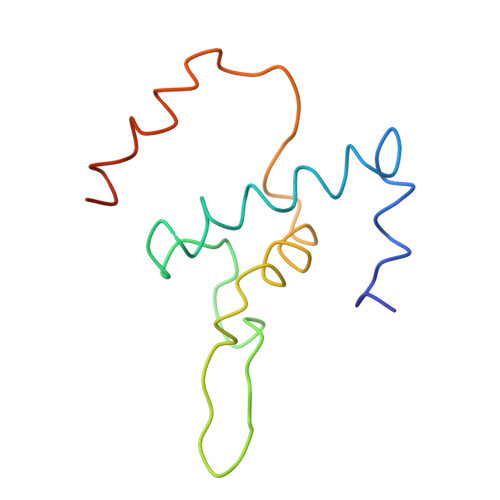
Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution.
Murakami, K.S., Masuda, S., Darst, S.A.(2002) Science 296: 1280-1284
- PubMed: 12016306
- DOI: https://doi.org/10.1126/science.1069594
- Primary Citation of Related Structures:
1L9U - PubMed Abstract:
The crystal structure of the initiating form of Thermus aquaticus RNA polymerase, containing core RNA polymerase (alpha2betabeta'omega) and the promoter specificity sigma subunit, has been determined at 4 angstrom resolution. Important structural features of the RNA polymerase and their roles in positioning sigma within the initiation complex are delineated, as well as the role played by sigma in modulating the opening of the RNA polymerase active-site channel. The two carboxyl-terminal domains of sigma are separated by 45 angstroms on the surface of the RNA polymerase, but are linked by an extended loop. The loop winds near the RNA polymerase active site, where it may play a role in initiating nucleotide substrate binding, and out through the RNA exit channel. The advancing RNA transcript must displace the loop, leading to abortive initiation and ultimately to sigma release.
- The Rockefeller University, 1230 York Avenue, New York, NY 10021, USA.
Organizational Affiliation: