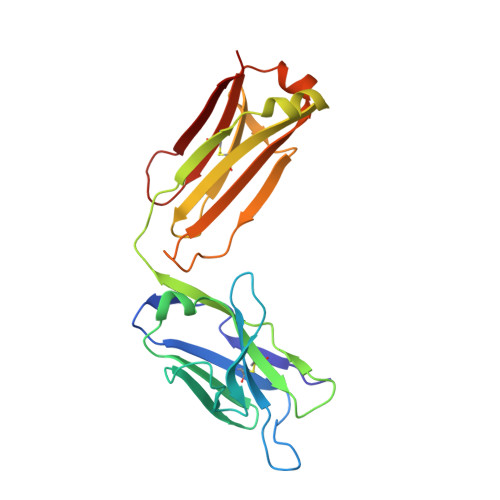
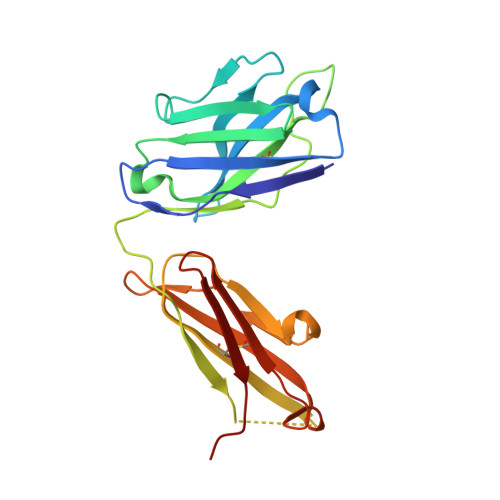
Crystal structure of the liganded anti-gibberellin A(4) antibody 4-B8(8)/E9 Fab fragment.
Murata, T., Fushinobu, S., Nakajima, M., Asami, O., Sassa, T., Wakagi, T., Yamaguchi, I.(2002) Biochem Biophys Res Commun 293: 489-496
- PubMed: 12054627
- DOI: https://doi.org/10.1016/S0006-291X(02)00225-5
- Primary Citation of Related Structures:
1KFA - PubMed Abstract:
Gibberellins, a class of plant hormones, consist of more than 120 members. Only a few of them are recognized by a receptor that remains unknown. The haptenic mouse monoclonal antibody, 4-B8(8)/E9, was generated against gibberellin A(4) (GA(4)) to recognize biologically active GA selectivity, and we attempted to confirm the binding properties between the antibody and GA(4). We carried out an X-ray crystallographic analysis of the 4-B8(8)/E9 Fab fragment complexed with GA(4) at a 2.8 A resolution by using the molecular replacement method. The crystal structure of the Fab fragment showed the typical immunoglobulin fold of the beta-barrel structure which is the common motif of all antibodies. A small hapten-combining site was made up of three heavy chain CDR loops. On the other hand, CDRs of the light chain did not interact directly with GA(4). The C/D rings of the GA(4) molecule were in van der Waals contact mainly with the aromatic side chain of Tyr100AH and Phe100BH of CDR-H3. The 3 beta-hydroxyl and 6 beta-carboxyl groups were, respectively, hydrogen-bonded to the main chain of Ala33H and to the Thr53H heavy chain.
- Department of Applied Biological Chemistry, Division of Agriculture and Agricultural Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan. tks@pgr1.ch.a.u-tokyo.ac.jp
Organizational Affiliation: