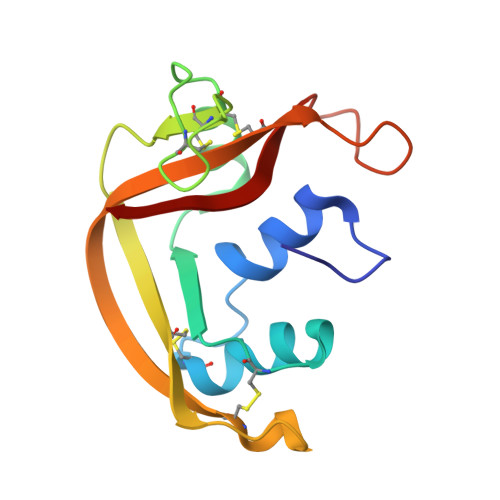
Crystallographic and functional studies of a modified form of eosinophil-derived neurotoxin (EDN) with novel biological activities.
Chang, C., Newton, D.L., Rybak, S.M., Wlodawer, A.(2002) J Mol Biology 317: 119-130
- PubMed: 11916383
- DOI: https://doi.org/10.1006/jmbi.2002.5406
- Primary Citation of Related Structures:
1K2A - PubMed Abstract:
The crystal structure of a post-translationally modified form of eosinophil-derived neurotoxin (EDN) with four extra residues on its N terminus ((-4)EDN) has been solved and refined at atomic resolution (1 A). Two of the extra residues can be placed unambiguously, while the density corresponding to two others is poor. The modified N terminus appears to influence the position of the catalytically important His129, possibly explaining the diminished catalytic activity of this variant. However, (-4)EDN has been shown to be cytotoxic to a Kaposi's sarcoma tumor cell line and other endothelial cell lines. Analysis of the structure and function suggests that the reason for cytotoxicity is most likely due to cellular recognition by the N-terminal extension, since the intrinsic activity of the enzyme is not sufficient for cytotoxicity and the N-terminal extension does not affect the conformation of EDN.
- Macromolecular Crystallography Laboratory, National Cancer Institute, Frederick, MD 21702, USA.
Organizational Affiliation: