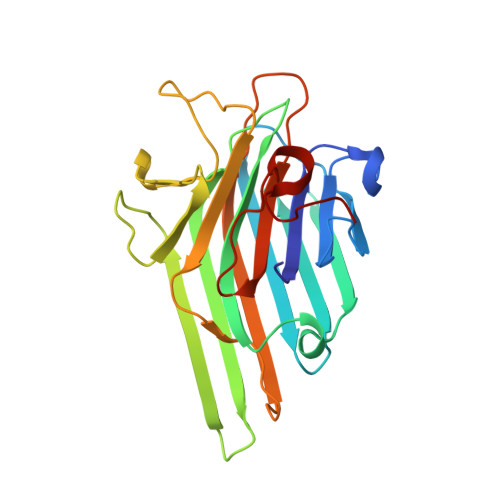
Plasticity in protein-peptide recognition: crystal structures of two different peptides bound to concanavalin A.
Jain, D., Kaur, K.J., Salunke, D.M.(2001) Biophys J 80: 2912-2921
- PubMed: 11371463
- DOI: https://doi.org/10.1016/S0006-3495(01)76256-X
- Primary Citation of Related Structures:
1JUI, 1JYC - PubMed Abstract:
The structures of concanavalin A (ConA) in complex with two carbohydrate-mimicking peptides, 10-mer (MYWYPYASGS) and 15-mer (RVWYPYGSYLTASGS) have been determined at 2.75 A resolution. In both crystal structures four independent peptide molecules bind to each of the crystallographically independent subunits of ConA tetramer. The peptides exhibit small but significant variability in conformations and interactions while binding to ConA. The crystal structure of another similar peptide, 12-mer (DVFYPYPYASGS), in complex with ConA has been determined (Jain, D., K. J. Kaur, B. Sundaravadivel, and D. M. Salunke. 2000. Structural and functional consequences of peptide-carbohydrate mimicry. J. Biol. Chem. 275:16098-16102). Comparison of the three complexes shows that the peptides bind to ConA at a common binding site, using different contacting residues and interactions depending on their sequence and the local environment at the binding site. The binding is also optimized by corresponding plasticity of the peptide binding site on ConA. The diversity in conformation and interactions observed here are in agreement with the structural leeway concerning plasticity of specific molecular recognition in biological processes. The adaptability of peptide-ConA interactions may also be correlated with the carbohydrate-mimicking property of these peptides.
- Structural Biology Unit, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi 110 067, India.
Organizational Affiliation: