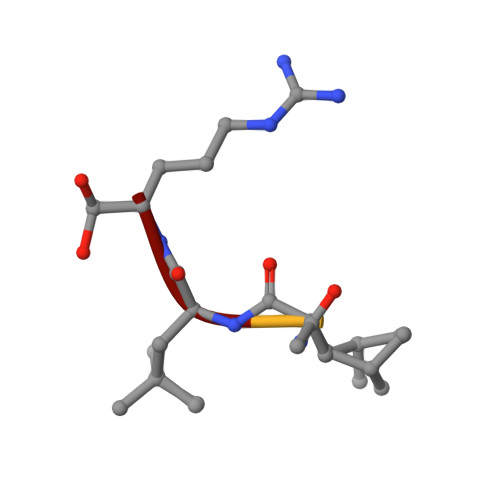
Two crystal structures of the leupeptin-trypsin complex.
Kurinov, I.V., Harrison, R.W.(1996) Protein Sci 5: 752-758
- PubMed: 8845765
- DOI: https://doi.org/10.1002/pro.5560050420
- Primary Citation of Related Structures:
1JRS, 1JRT - PubMed Abstract:
Three-dimensional structures of trypsin with the reversible inhibitor leupeptin have been determined in two different crystal forms. The first structure was determined at 1.7 A resolution with R-factor = 17.7% in the trigonal crystal space group P3(1)21, with unit cell dimensions of a = b = 55.62 A, c = 110.51 A. The second structure was determined at a resolution of 1.8 A with R-factor = 17.5% in the orthorhombic space group P2(1)2(1)2(1), with unit cell dimensions of a = 63.69 A, b = 69.37 A, c = 63.01 A. The overall protein structure is very similar in both crystal forms, with RMS difference for main-chain atoms of 0.27 A. The leupeptin backbone forms four hydrogen bonds with trypsin and a fifth hydrogen bond interaction is mediated by a water molecule. The aldehyde carbonyl of leupeptin forms a covalent bond of 1.42 A length with side-chain oxygen of Ser-195 in the active site. The reaction of trypsin with leupeptin proceeds through the formation of stable tetrahedral complex in which the hemiacetal oxygen atom is pointing out of the oxyanion hole and forming a hydrogen bond with His-57.
- Department of Pharmacology, Thomas Jefferson University, Philadelphia, Pennsylvania 19107, USA.
Organizational Affiliation: