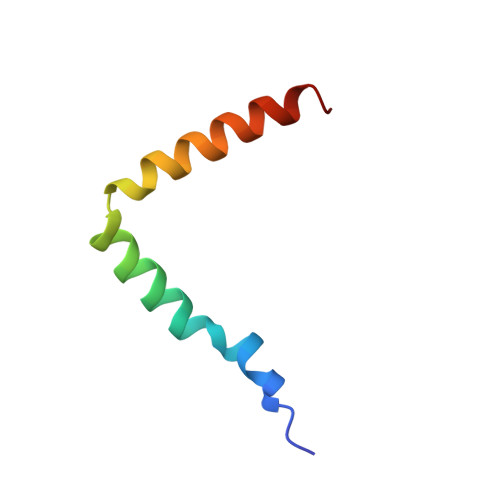
Structure of the Rhodobacter sphaeroides light-harvesting 1 beta subunit in detergent micelles.
Sorgen, P.L., Cahill, S.M., Krueger-Koplin, R.D., Krueger-Koplin, S.T., Schenck, C.C., Girvin, M.E.(2002) Biochemistry 41: 31-41
- PubMed: 11772000
- DOI: https://doi.org/10.1021/bi011576j
- Primary Citation of Related Structures:
1JO5 - PubMed Abstract:
The light harvesting 1 antenna (LH1) complex from Rhodobacter sphaeroides funnels excitation energy to the photosynthetic reaction center. Our ultimate goal is to build up the structure of LH1 from structures of its individual subunits, much as the antenna can self-assemble from its components in membrane-mimicking detergent micelles. The beta subunit adopts a nativelike conformation in Zwittergent 3:12 micelles as demonstrated by its ability to take the first step of assembly, binding BChl a. Multidimensional NMR spectroscopy shows that the beta subunit folds as a helix((L12-S25))-hinge((G26-W28))-helix((L29-W44)) structure with the helical regions for the 10 lowest-energy structures having backbone rmsds of 0.26 and 0.24 A, respectively. Mn(2+) relaxation data and the protein-detergent NOE pattern show the C-terminal helix embedded in the micelle and the N-terminal helix lying along the detergent micelle surface with a 60 degrees angle between their long axes. (15)N relaxation data for residues L12-W44 are typical of a well-ordered protein with a correlation time of 8.25 +/- 2.1 ns. The presence of the hinge region placing the N-terminal helix along the membrane surface may be the structural feature responsible for the functional differences observed between the LH1 and LH2 beta subunits.
- Department of Biochemistry, Albert Einstein College of Medicine, Bronx, New York 10461, USA.
Organizational Affiliation: