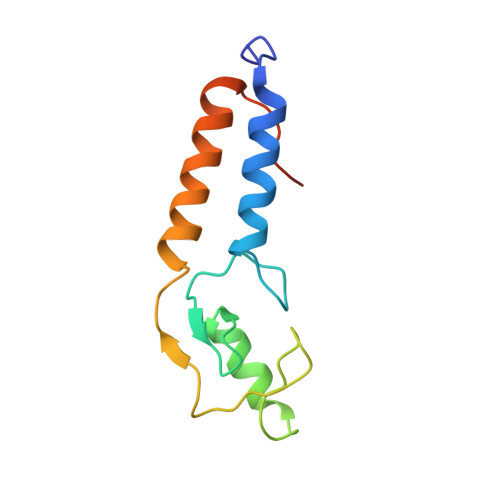
Structure of a BRCA1-BARD1 heterodimeric RING-RING complex.
Brzovic, P.S., Rajagopal, P., Hoyt, D.W., King, M.C., Klevit, R.E.(2001) Nat Struct Biol 8: 833-837
- PubMed: 11573085
- DOI: https://doi.org/10.1038/nsb1001-833
- Primary Citation of Related Structures:
1JM7 - PubMed Abstract:
The RING domain of the breast and ovarian cancer tumor suppressor BRCA1 interacts with multiple cognate proteins, including the RING protein BARD1. Proper function of the BRCA1 RING domain is critical, as evidenced by the many cancer-predisposing mutations found within this domain. We present the solution structure of the heterodimer formed between the RING domains of BRCA1 and BARD1. Comparison with the RING homodimer of the V(D)J recombination-activating protein RAG1 reveals the structural diversity of complexes formed by interactions between different RING domains. The BRCA1-BARD1 structure provides a model for its ubiquitin ligase activity, illustrates how the BRCA1 RING domain can be involved in associations with multiple protein partners and provides a framework for understanding cancer-causing mutations at the molecular level.
- Department of Biochemistry and Biomolecular Structure Center, University of Washington, Seattle, Washington 98195-7742, USA.
Organizational Affiliation: