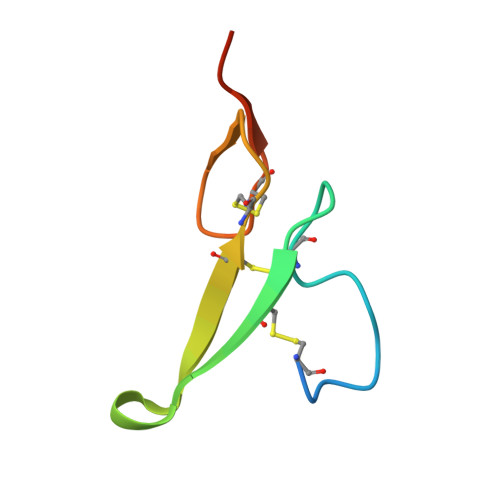
Crystal structure of human epidermal growth factor and its dimerization
Lu, H.S., Chai, J.J., Li, M., Huang, B.R., He, C.H., Bi, R.C.(2001) J Biological Chem 276: 34913-34917
- PubMed: 11438527
- DOI: https://doi.org/10.1074/jbc.M102874200
- Primary Citation of Related Structures:
1JL9 - PubMed Abstract:
Epidermal growth factor (EGF) is a typical growth-stimulating peptide and functions by binding to specific cell-surface receptors and inducing dimerization of the receptors. Little is known about the molecular mechanism of EGF-induced dimerization of EGF receptors. The crystal structure of human EGF has been determined at pH 8.1. There are two human EGF molecules A and B in the asymmetric unit of the crystals, which form a potential dimer. Importantly, a number of residues known to be indispensable for EGF binding to its receptor are involved in the interface between the two EGF molecules, suggesting a crucial role of EGF dimerization in the EGF-induced dimerization of receptors. In addition, the crystal structure of EGF shares the main features of the NMR structure of mouse EGF determined at pH 2.0, but structural comparisons between different models have revealed new detailed features and properties of the EGF structure.
- Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.
Organizational Affiliation: