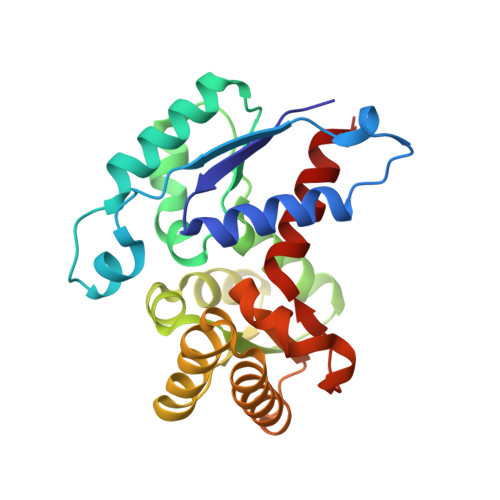
Crystal structure of aspartate racemase from Pyrococcus horikoshii OT3 and its implications for molecular mechanism of PLP-independent racemization.
Liu, L., Iwata, K., Kita, A., Kawarabayasi, Y., Yohda, M., Miki, K.(2002) J Mol Biology 319: 479-489
- PubMed: 12051922
- DOI: https://doi.org/10.1016/S0022-2836(02)00296-6
- Primary Citation of Related Structures:
1JFL - PubMed Abstract:
There exists a d-enantiomer of aspartic acid in lactic acid bacteria and several hyperthermophilic archaea, which is biosynthesized from the l-enantiomer by aspartate racemase. Aspartate racemase is a representative pyridoxal 5'-phosphate (PLP)-independent amino acid racemase. The "two-base" catalytic mechanism has been proposed for this type of racemase, in which a pair of cysteine residues are utilized as the conjugated catalytic acid and base. We have determined the three-dimensional structure of aspartate racemase from the hyperthermophilic archaeum Pyrococcus horikoshii OT3 at 1.9 A resolution by X-ray crystallography and refined it to a crystallographic R factor of 19.4% (R(free) of 22.2%). This is the first structure reported for aspartate racemase, indeed for any amino acid racemase from archaea. The crystal structure revealed that this enzyme forms a stable dimeric structure with a strong three-layered inter-subunit interaction, and that its subunit consists of two structurally homologous alpha/beta domains, each containing a four-stranded parallel beta-sheet flanked by six alpha-helices. Two strictly conserved cysteine residues (Cys82 and Cys194), which have been shown biochemically to act as catalytic acid and base, are located on both sides of a cleft between the two domains. The spatial arrangement of these two cysteine residues supports the "two-base" mechanism but disproves the previous hypothesis that the active site of aspartate racemase is located at the dimeric interface. The structure revealed a unique pseudo mirror-symmetry in the spatial arrangement of the residues around the active site, which may explain the molecular recognition mechanism of the mirror-symmetric aspartate enantiomers by the non-mirror-symmetric aspartate racemase.
- Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan.
Organizational Affiliation: