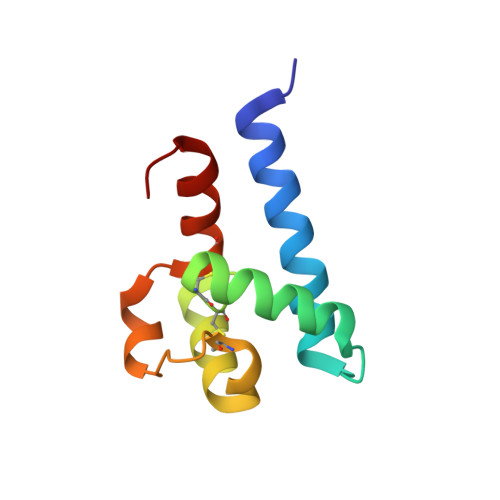
Subunit Association and Conformational Flexibility in the Head-subdomain of Human CD81 Large Extracellular Loop.
Kitadokoro, K., Ponassi, M., Galli, G., Petracca, R., Falugi, F., Grandi, G., Bolognesi, M.(2002) Biol Chem 383: 1447-1452
- PubMed: 12437138
- DOI: https://doi.org/10.1515/BC.2002.164
- Primary Citation of Related Structures:
1IV5 - PubMed Abstract:
The large extracellular loop of human CD81, a tetraspanin mediating hepatitis C virus envelope protein E2 binding to human cells, has been crystallized in a hexagonal form. The three-dimensional structure, solved and refined at 2.6 A resolution (R-factor = 22.8%), shows that the protein adopts a dimeric assembly, based on an association interface built up by tetraspanin-conserved residues. Structural comparisons with the tertiary structure of human CD81 large extracellular loop, previously determined in a different crystal form, show marked conformational fluctuations in the molecular regions thought to be involved in binding to the viral protein, suggesting rules for recognition and assembly within the tetraspan web.
- Department of Physics-INFM, Center of Excellence for Biomedical Research, University of Genoa, Italy.
Organizational Affiliation: