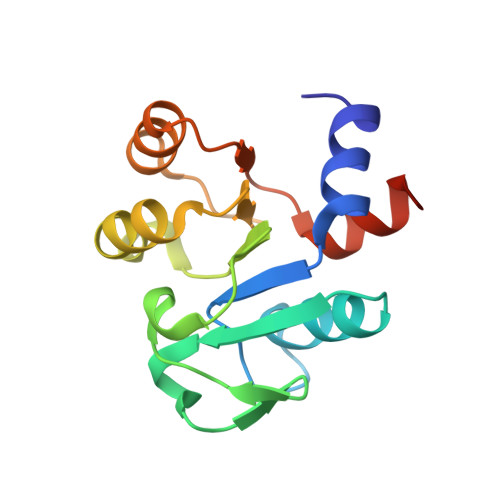
Structure of a conserved CoA-binding protein synthesized by a cell-free system.
Wada, T., Shirouzu, M., Terada, T., Ishizuka, Y., Matsuda, T., Kigawa, T., Kuramitsu, S., Park, S.Y., Tame, J.R., Yokoyama, S.(2003) Acta Crystallogr D Biol Crystallogr 59: 1213-1218
- PubMed: 12832765
- DOI: https://doi.org/10.1107/s0907444903010515
- Primary Citation of Related Structures:
1IUK, 1IUL - PubMed Abstract:
TT1466 is a hypothetical protein from the extremely thermophilic bacterium Thermus thermophilus HB8 and is highly conserved in bacteria and archaea. The selenomethionyl protein was synthesized by a cell-free system and the crystal structure was determined at 2.0 A by MAD phasing. A native crystal was used for structure refinement to 1.7 A. The structure is highly homologous to that of the CoA-binding domain of the succinyl-CoA synthetase from Escherichia coli, despite the protein having only 14% sequence identity to this domain. An isothermal titration calorimetry experiment was performed to investigate whether TT1466 binds CoA and revealed high-affinity CoA binding of TT1466.
- RIKEN Genomic Sciences Center, 1-7-22 Suehiro-cho, Tsurumi, Yokohama 230-0045, Japan.
Organizational Affiliation: