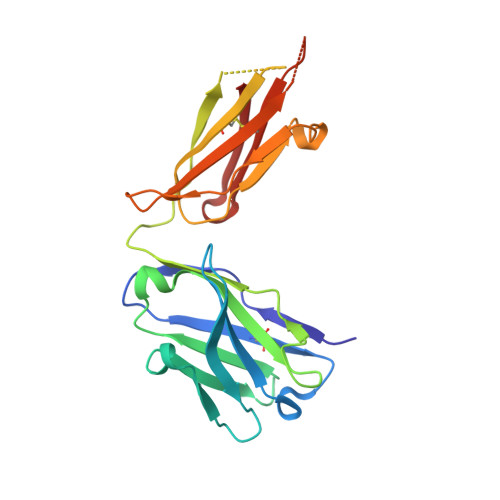
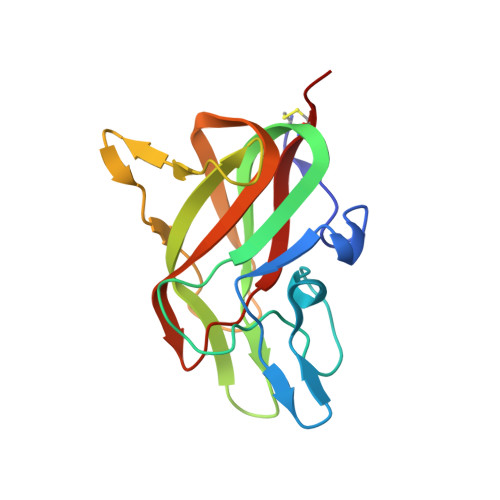
Structure of a factor VIII C2 domain-immunoglobulin G4kappa Fab complex: identification of an inhibitory antibody epitope on the surface of factor VIII.
Spiegel Jr., P.C., Jacquemin, M., Saint-Remy, J.M., Stoddard, B.L., Pratt, K.P.(2001) Blood 98: 13-19
- PubMed: 11418455
- DOI: https://doi.org/10.1182/blood.v98.1.13
- Primary Citation of Related Structures:
1IQD - PubMed Abstract:
The development of an immune response to infused factor VIII is a complication affecting many patients with hemophilia A. Inhibitor antibodies bind to antigenic determinants on the factor VIII molecule and block its procoagulant activity. A patient-derived inhibitory immunoglobulin G4kappa antibody (BO2C11) produced by an immortalized memory B-lymphocyte cell line interferes with the binding of factor VIII to phospholipid surfaces and to von Willebrand factor. The structure of a Fab fragment derived from this antibody complexed with the factor VIII C2 domain was determined at 2.0 A resolution. The Fab interacts with solvent-exposed basic and hydrophobic side chains that form a membrane-association surface of factor VIII. This atomic resolution structure suggests a variety of amino acid substitutions in the C2 domain of factor VIII that might prevent the binding of anti-C2 inhibitor antibodies without significantly compromising the procoagulant functions of factor VIII.
- Graduate Program in Biomolecular Structure and Design, University of Washington, and Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Organizational Affiliation: