Crystal structure of human PEDF, a potent anti-angiogenic and neurite growth-promoting factor.
Simonovic, M., Gettins, P.G., Volz, K.(2001) Proc Natl Acad Sci U S A 98: 11131-11135
- PubMed: 11562499
- DOI: https://doi.org/10.1073/pnas.211268598
- Primary Citation of Related Structures:
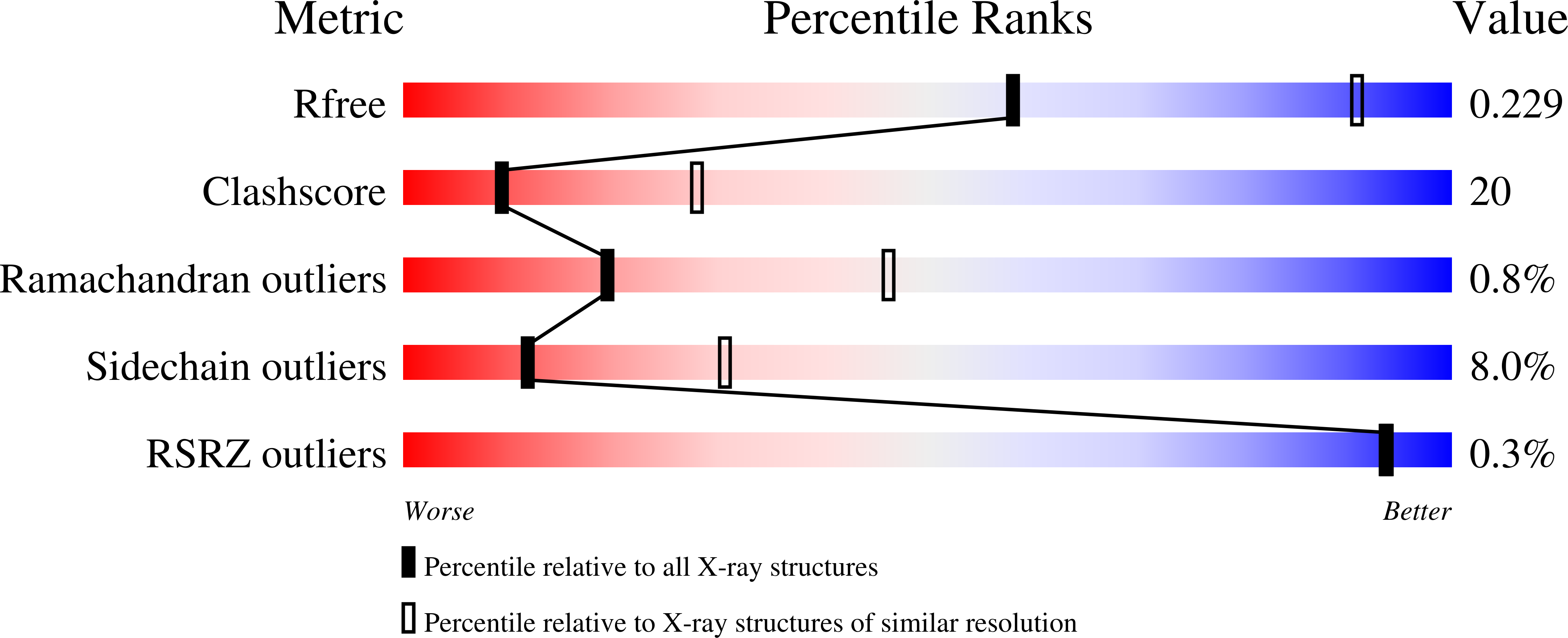
1IMV - PubMed Abstract:
Pigment epithelium-derived factor (PEDF), a noninhibitory member of the serpin superfamily, is the most potent inhibitor of angiogenesis in the mammalian ocular compartment. It also has neurotrophic activity, both in the retina and in the central nervous system, and is highly up-regulated in young versus senescent fibroblasts. To provide a structural basis for understanding its many biological roles, we have solved the crystal structure of glycosylated human PEDF to 2.85 A. The structure revealed the organization of possible receptor and heparin-binding sites, and showed that, unlike any other previously characterized serpin, PEDF has a striking asymmetric charge distribution that might be of functional importance. These results provide a starting point for future detailed structure/function analyses into possible mechanisms of PEDF action that could lead to development of therapeutics against uncontrolled angiogenesis.
- Department of Biochemistry, College of Medicine, University of Illinois, Chicago, IL 60612-7334, USA.
Organizational Affiliation: