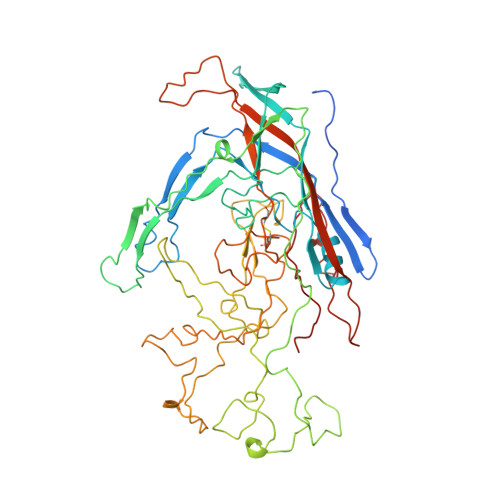
Structural analysis of a mutation in canine parvovirus which controls antigenicity and host range.
Llamas-Saiz, A.L., Agbandje-McKenna, M., Parker, J.S., Wahid, A.T., Parrish, C.R., Rossmann, M.G.(1996) Virology 225: 65-71
- PubMed: 8918534
- DOI: https://doi.org/10.1006/viro.1996.0575
- Primary Citation of Related Structures:
1IJS - PubMed Abstract:
A single mutation in canine parvovirus (CPV) of VP2 residue 300 from alanine to aspartic acid causes a loss of canine host range and alters the antigenic properties of the virus. The three-dimensional structure of this mutant has been solved to 3.25 A resolution. Crystals of full particles were triclinic, with cell dimensions of a = 267.6, b = 268.5, c = 274.3 A. alpha = 61.9, beta = 62.6, and gamma = 60.2 degrees. The native structure of CPV was used as an initial model. Phases were improved by real-space electron density averaging. In spite of the relative low percentage of observed reflections (32.5% of the data between 15.0 and 3.25 A resolution), the presence of 60-fold noncrystallographic redundancy allowed the averaging procedure to converge smoothly. The mutant aspartic acid at residue 300 forms a salt bridge with Arg81 in an icosahedrally threefold-related subunit, inducing local changes within the antigenic site B on the CPV surface. In addition, the loop between residues 359 and 374 adopts a conformation similar to that displayed by feline panleukopenia virus. The ability of the Ala300-->Asp mutant to evade antibody binding can be associated with the change of charge distribution and structure in the antigenic binding site. The variation in host range behavior may be due to the increased stability as a result of formation of the salt bridge between adjacent subunits.
- Department of Biological Sciences, Purdue University, West Lafayette, Indiana 47907-1392, USA.
Organizational Affiliation: