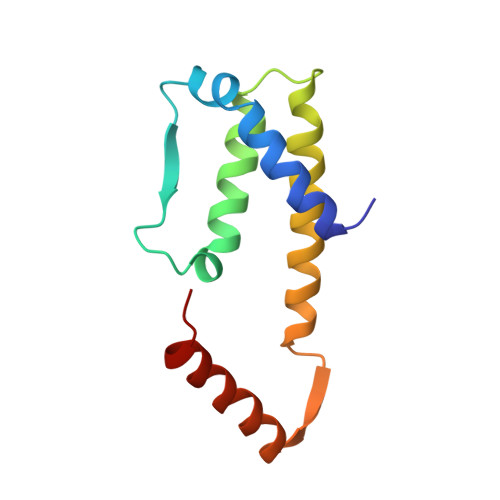
A novel dimer configuration revealed by the crystal structure at 2.4 A resolution of human interleukin-5.
Milburn, M.V., Hassell, A.M., Lambert, M.H., Jordan, S.R., Proudfoot, A.E., Graber, P., Wells, T.N.(1993) Nature 363: 172-176
- PubMed: 8483502
- DOI: https://doi.org/10.1038/363172a0
- Primary Citation of Related Structures:
1HUL - PubMed Abstract:
Interleukin-5 (IL-5) is a lineage-specific cytokine for eosinophilpoiesis and plays an important part in diseases associated with increased eosinophils, such as asthma. Human IL-5 is a disulphide-linked homodimer with 115 amino-acid residues in each chain. The crystal structure at 2.4 A resolution reveals a novel two-domain structure, with each domain showing a striking similarity to the cytokine fold found in granulocyte macrophage and macrophage colony-stimulating factors, IL-2 (ref. 5), IL-4 (ref. 6), and human and porcine growth hormones. IL-5 is unique in that each domain requires the participation of two chains. The IL-5 structure consists of two left-handed bundles of four helices laid end to end and two short beta-sheets on opposite sides of the molecule. Surprisingly, the C-terminal strand and helix of one chain complete a bundle of four helices and a beta-sheet with the N-terminal three helices and one strand of the other chain. The structure of IL-5 provides a molecular basis for the design of antagonists and agonists that would delineate receptor recognition determinants critical in signal transduction. This structure determination extends the family of the cytokine bundle of four helices and emphasizes its fundamental significance and versatility in recognizing its receptor.
- Laboratory of Molecular Medicine, Children's Hospital/Ender 673, Boston, Massachusetts 02115-5737.
Organizational Affiliation: