Synthesis, characterization, and application of cy-dye- and alexa-dye-labeled hongotoxin(1) analogues. The first high affinity fluorescence probes for voltage-gated K+ channels.
Pragl, B., Koschak, A., Trieb, M., Obermair, G., Kaufmann, W.A., Gerster, U., Blanc, E., Hahn, C., Prinz, H., Schutz, G., Darbon, H., Gruber, H.J., Knaus, H.G.(2002) Bioconjug Chem 13: 416-425
- PubMed: 12009929
- DOI: https://doi.org/10.1021/bc015543s
- Primary Citation of Related Structures:
1HLY - PubMed Abstract:
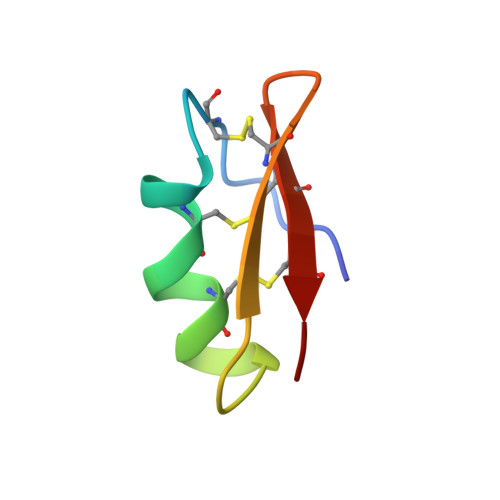
Hongotoxin(1) (HgTX(1)), a 39-residue peptide recently isolated from the venom of Centruroides limbatus, blocks the voltage-gated K+ channels K(v)1.1, K(v)1.2, and K(v)1.3 at picomolar toxin concentrations (Koschak, A., Bugianesi, R. M., Mitterdorfer, J., Kaczorowski, G. J., Garcia, M. L., and Knaus, H. G. (1998) J. Biol. Chem. 273, 2639-2644). In this report, we determine the three-dimensional structure of HgTX(1) using NMR spectroscopy (PDB-code: 1HLY). HgTX(1) was found to possess a structure similar to previously characterized K+ channel toxins (e.g. margatoxin) consisting of a three-stranded antiparallel beta-sheet (residues 2-4, 26-30, and 33-37) and a helical conformation (part 3(10) helix and part alpha helix; residues 10-20). Due to the importance of residue Lys-28 for high-affinity interaction with the respective channels, lysine-reactive fluorescence dyes cannot be used to label wild-type HgTX(1). On the basis of previous studies (see above) and our NMR data, a HgTX(1) mutant (HgTX(1)-A19C) was engineered, expressed, and purified. HgTX(1)-A19C-SH was labeled using sulfhydryl-reactive Cy3-, Cy5-, and Alexa-dyes. Pharmacological characterization of fluorescently labeled HgTX(1)-A19C in radioligand binding studies indicated that these hongotoxin(1) analogues retain high-affinity for voltage-gated K+ channels and a respective pharmacological profile. Cy3- and Alexa-dye-labeled hongotoxin(1) analogues were used to investigate the localization of K+ channels in brain sections. The distribution of toxin binding closely follows the distribution of K(v)1.2 immunoreactivity with the highest expression levels in the cerebellar Purkinje cell layer. Taken together, these results demonstrate that fluorescently labeled HgTX(1) analogues comprise novel probes to characterize a subset of voltage-gated K+ channels.
- Institut für Biochemische Pharmakologie, Universität Innsbruck, Peter Mayr-Strasse 1, A-6020, Austria.
Organizational Affiliation: