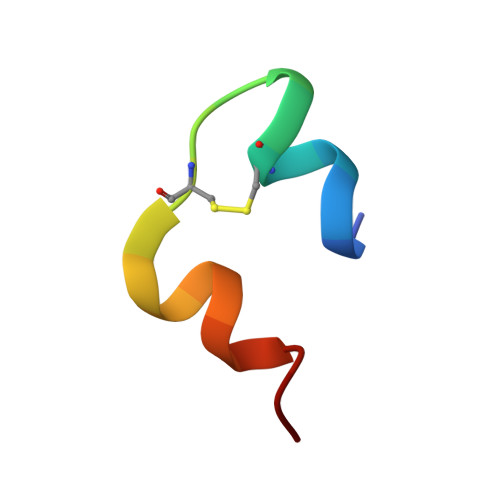
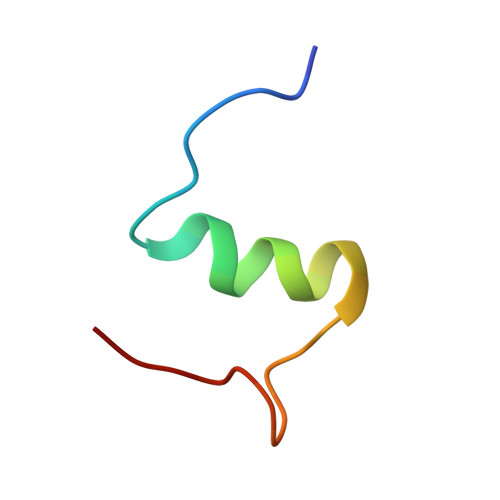
Paradoxical structure and function in a mutant human insulin associated with diabetes mellitus.
Hua, Q.X., Shoelson, S.E., Inouye, K., Weiss, M.A.(1993) Proc Natl Acad Sci U S A 90: 582-586
- PubMed: 8421693
- DOI: https://doi.org/10.1073/pnas.90.2.582
- Primary Citation of Related Structures:
1HIQ - PubMed Abstract:
The solution structure of a diabetes-associated mutant human insulin (insulin Los Angeles; PheB24-->Ser) was determined by 13C-edited NMR spectroscopy and distance-geometry/simulated annealing calculations. Among vertebrate insulins PheB24 is invariant, and in crystal structures the aromatic ring appears to anchor the putative receptor-binding surface through long-range packing interactions in the hydrophobic core. B24 substitutions are of particular interest in relation to the mechanism of receptor binding. In one analogue ([GlyB24]insulin), partial unfolding of the B chain has been observed with paradoxical retention of near-native bioactivity. The present study of [SerB24]insulin extends this observation: relative to [GlyB24]insulin, near-native structure is restored despite significant loss of function. To our knowledge, our results provide the first structural study of a diabetes-associated mutant insulin and support the hypothesis that insulin undergoes a change in conformation on receptor binding.
- Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115.
Organizational Affiliation: