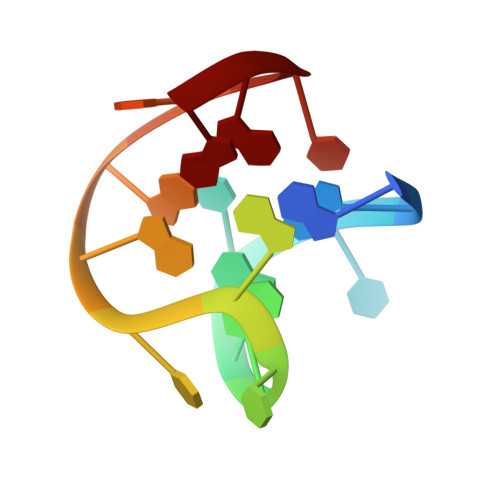
An ambiguous structure of a DNA 15-mer thrombin complex.
Padmanabhan, K., Tulinsky, A.(1996) Acta Crystallogr D Biol Crystallogr 52: 272-282
- PubMed: 15299700
- DOI: https://doi.org/10.1107/S0907444995013977
- Primary Citation of Related Structures:
1HAO, 1HAP - PubMed Abstract:
The structure of a complex between thrombin and a GGTTGGTGTGGTTGG DNA 15-mer has been analyzed crystallographically. The solution NMR structure of the 15-mer has two stacked G-quartets similar to that found in the previous X-ray structure determination of the 15-mer-thrombin complex [Padmanabhan, Padmanabhan, Ferrara, Sadler & Tulinsky (1993). J. Biol. Chem. 268, 17651-17654]; the strand polarity, however, is reversed from that of the crystallographic structure. The structure of the complex here has been redetermined with better diffraction data confirming the previous crystallographic structure but also indicating that the NMR solution structure fits equally well. Both 15-mer complex structures refined to an R value of about 0.16 presenting a disconcerting ambiguity. Since the two 15-mer structures associate with thrombin in different ways (through the TGT loop in the X-ray and TT loop in the NMR model), other independent lines of physical or chemical evidence are required to resolve the ambiguity.
- Department of Chemistry, Michigan State University, East Lansing 48824, USA.
Organizational Affiliation: