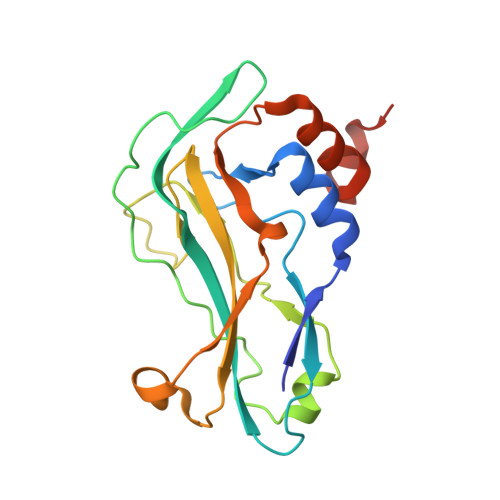
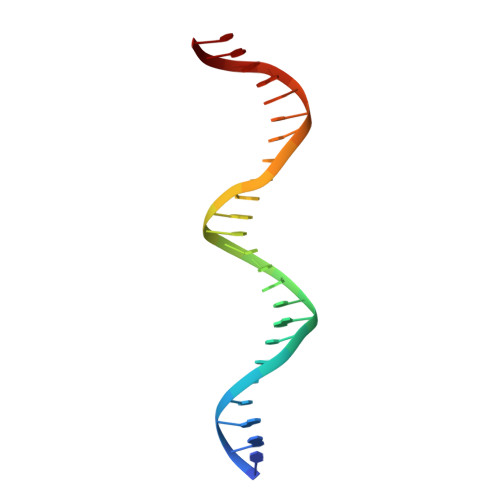
Structure of the DNA-Bound T-Box Domain of Human Tbx3, a Transcription Factor Responsible for Ulnar- Mammary Syndrome
Coll, M., Seidman, J.G., Muller, C.W.(2002) Structure 10: 343
- PubMed: 12005433
- DOI: https://doi.org/10.1016/s0969-2126(02)00722-0
- Primary Citation of Related Structures:
1H6F - PubMed Abstract:
T-box genes encode transcription factors involved in morphogenesis and organogenesis of vertebrates and invertebrates. Mutations in human T-box genes TBX3, TBX5, and TBX1 cause severe genetic disorders known as Ulnar-Mammary syndrome (UMS), Holt-Oram syndrome (HOS), and DiGeorge syndrome, respectively. The crystal structure of the T-box domain of the first human T-box transcription factor, TBX3, in complex with DNA at 1.7 A resolution explains structural consequences of T-box domain point mutations observed in UMS and HOS patients. Comparison with the structure of the T-box domain from Xenopus laevis (Xbra) bound to DNA shows differences in several secondary structure elements and in the quaternary structure of the two complexes. TBX3 independently recognizes the two binding sites present in the palindromic DNA duplex, whereas in Xbra, binding to the palindrome is stabilized through interactions between the two monomers. The different quaternary structures suggest different DNA binding modes for T-box transcription factors.
- European Molecular Biology Laboratory, Grenoble Outstation, France.
Organizational Affiliation: