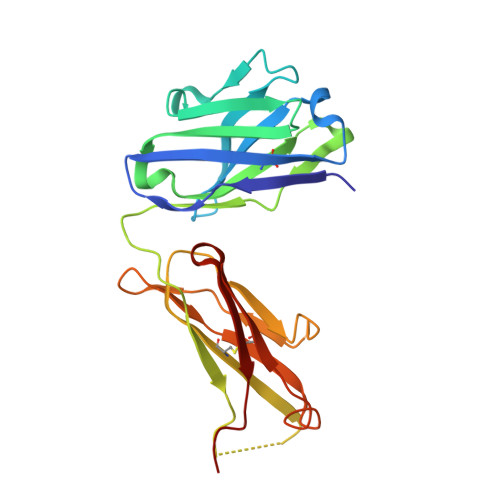
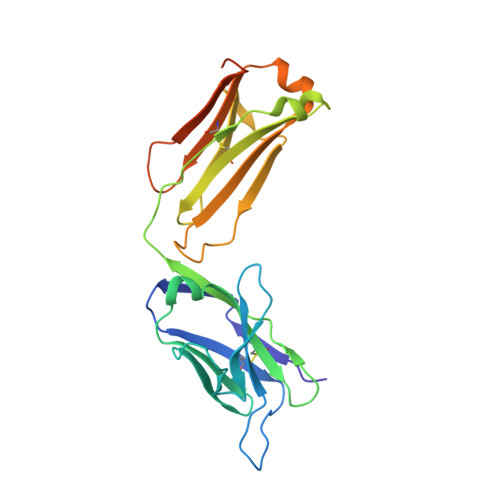
Structural and Functional Characterisation of a Monoclonal Antibody Specific for the Pres1 Region of Hepatitis B Virus
Pizarro, J.C., Vulliez-Le-Normand, B., Riottot, M.M., Budkowska, A., Bentley, G.A.(2001) FEBS Lett 509: 463
- PubMed: 11749974
- DOI: https://doi.org/10.1016/s0014-5793(01)03190-8
- Primary Citation of Related Structures:
1H3P - PubMed Abstract:
The monoclonal antibody 5a19, raised against the ay serotype of hepatitis B virus, binds to the segment of the preS1 region comprising residues 37-43, which is implicated in attachment of the virus to hepatocytes. The dissociation constant, derived from kinetic studies using surface plasmon resonance techniques, is in the low nanomolar range. The nucleotide sequence of the variable domains has been determined and the corresponding germ-line genes have been identified. The three-dimensional structure of the Fab fragment has been determined by X-ray crystallography to 2.6 A resolution.
- Unité d' Immunologie Structurale, Institut Pasteur, Paris, France.
Organizational Affiliation: