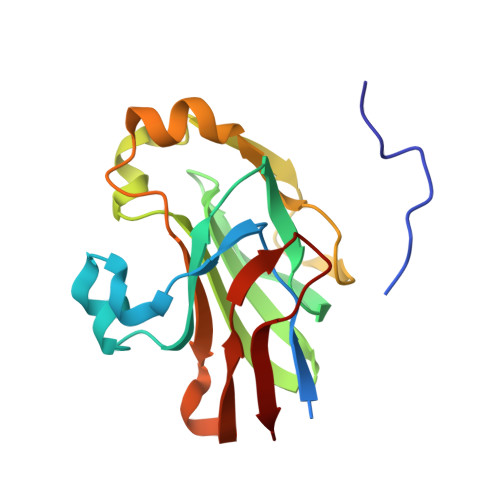
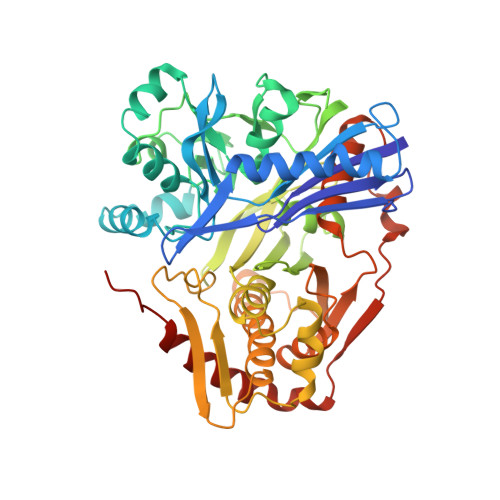
Structure of the regulatory complex of Escherichia coli IIIGlc with glycerol kinase.
Hurley, J.H., Faber, H.R., Worthylake, D., Meadow, N.D., Roseman, S., Pettigrew, D.W., Remington, S.J.(1993) Science 259: 673-677
- PubMed: 8430315
- Primary Citation of Related Structures:
1GLA, 1GLB - PubMed Abstract:
The phosphocarrier protein IIIGlc is an integral component of the bacterial phosphotransferase (PTS) system. Unphosphorylated IIIGlc inhibits non-PTS carbohydrate transport systems by binding to diverse target proteins. The crystal structure at 2.6 A resolution of one of the targets, glycerol kinase (GK), in complex with unphosphorylated IIIGlc, glycerol, and adenosine diphosphate was determined. GK contains a region that is topologically identical to the adenosine triphosphate binding domains of hexokinase, the 70-kD heat shock cognate, and actin. IIIGlc binds far from the catalytic site of GK, indicating that long-range conformational changes mediate the inhibition of GK by IIIGlc. GK and IIIGlc are bound by hydrophobic and electrostatic interactions, with only one hydrogen bond involving an uncharged group. The phosphorylation site of IIIGlc, His90, is buried in a hydrophobic environment formed by the active site region of IIIGlc and a 3(10) helix of GK, suggesting that phosphorylation prevents IIIGlc binding to GK by directly disrupting protein-protein interactions.
- Institute of Molecular Biology, University of Oregon, Eugene 97403.
Organizational Affiliation: