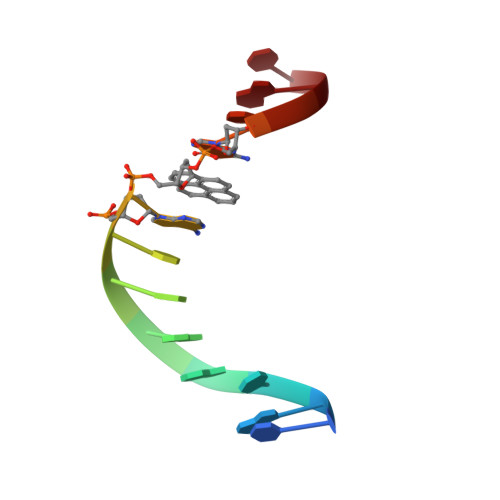
Integrity of duplex structures without hydrogen bonding: DNA with pyrene paired at abasic sites
Smirnov, S., Matray, T.J., Kool, E.T., de los Santos, C.(2002) Nucleic Acids Res 30: 5561-5569
- PubMed: 12490724
- DOI: https://doi.org/10.1093/nar/gkf688
- Primary Citation of Related Structures:
1FZL, 1FZS - PubMed Abstract:
DNA polymerases specifically insert the hydrophobic pyrene deoxynucleotide (P) opposite tetrahydrofuran (F), an stable abasic site analog, and DNA duplexes containing this non-hydrogen-bonded pair possess a high degree of thermodynamic stability. These observations support the hypothesis that steric complementarity and stacking interactions may be sufficient for maintaining stability of DNA structure and specificity of DNA replication, even in the absence of hydrogen bonds across the base pair. Here we report the NMR characterization and structure determination of two DNA molecules containing pyrene residues. The first is a 13mer duplex with a pyrene.tetrahydrofuran pair (P.F pair) at the ninth position and the second mimics a replication intermediate right after incorporation of a pyrene nucleoside opposite an abasic site. Our data indicate that both molecules adopt right-handed helical conformations with Watson- Crick alignments for all canonical base pairs. The pyrene ring stays inside the helix close to its baseless partner in both molecules. The single-stranded region of the replication intermediate folds back over the opposing strand, sheltering the hydrophobic pyrene moiety from water exposure. The results support the idea that the stability and replication of a P.F pair is due to its ability to mimic Watson-Crick structure.
- Department of Pharmacological Sciences, State University of New York at Stony Brook, Stony Brook, NY 11794-8651, USA.
Organizational Affiliation: