Solution structure of the N-terminal RNP domain of U1A protein: the role of C-terminal residues in structure stability and RNA binding.
Avis, J.M., Allain, F.H., Howe, P.W., Varani, G., Nagai, K., Neuhaus, D.(1996) J Mol Biology 257: 398-411
- PubMed: 8609632
- DOI: https://doi.org/10.1006/jmbi.1996.0171
- Primary Citation of Related Structures:
1FHT - PubMed Abstract:
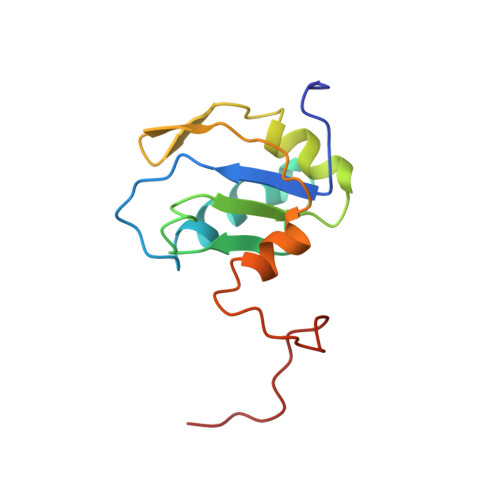
The solution structure of a fragment of the human U1A spliceosomal protein containing residues 2 to 117 (U1A117) determined using multi-dimensional heteronuclear NMR is presented. The C-terminal region of the molecule is considerably more ordered in the free protein than thought previously and its conformation is different from that seen in the crystal structure of the complex with U1 RNA hairpin II. The residues between Asp90 and Lys98 form an alpha-helix that lies across the beta-sheet, with residues IIe93, IIe94 and Met97 making contacts with Leu44, Phe56 and IIe58. This interaction prevents solvent exposure of hydrophobic residues on the surface of the beta-sheet, thereby stabilising the protein. Upon RNA binding, helix C moves away from this position, changing its orientation by 135 degrees to allow Tyr13, Phe56 and Gln54 to stack with bases of the RNA, and also allowing Leu44 to contact the RNA. The new position of helix C in the complex with RNA is stabilised by hydrophobic interactions from IIe93 and IIe94 to IIe58, Leu 41, Val62 and His 10, as well as a hydrogen bond between Ser91 and Thr11. The movement of helix C mainly involves changes in the main-chain torsion angles of Thr89, Asp90 and Ser91, the helix thereby acting as a "lid" over the RNA binding surface.
- MRC Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: