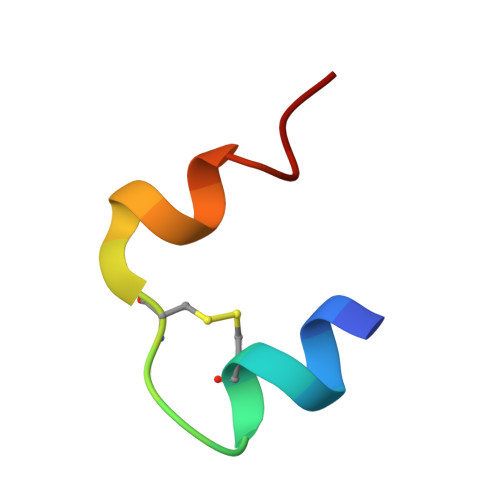
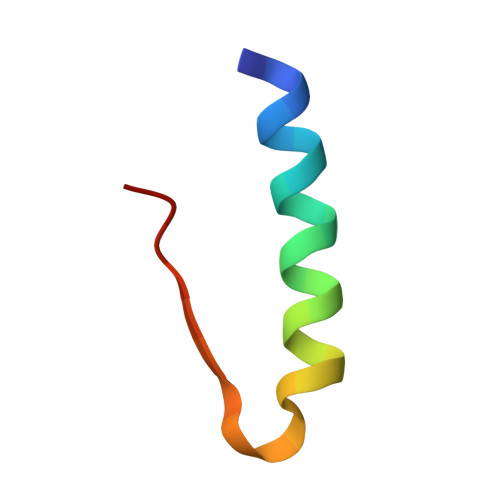
R6 hexameric insulin complexed with m-cresol or resorcinol.
Smith, G.D., Ciszak, E., Magrum, L.A., Pangborn, W.A., Blessing, R.H.(2000) Acta Crystallogr D Biol Crystallogr 56: 1541-1548
- PubMed: 11092919
- DOI: https://doi.org/10.1107/s0907444900012749
- Primary Citation of Related Structures:
1EV3, 1EV6, 1EVR - PubMed Abstract:
The structures of three R(6) human insulin hexamers have been determined. Crystals of monoclinic m-cresol-insulin, monoclinic resorcinol-insulin and rhombohedral m-cresol-insulin diffracted to 1. 9, 1.9 and 1.78 A, respectively, and have been refined to residuals of 0.195, 0.179 and 0.200, respectively. In all three structures, a phenolic derivative is found to occupy the phenolic binding site, where it forms hydrogen bonds to the carbonyl O atom of CysA6 and the N atom of CysA11. Two additional phenolic derivative binding sites were identified within or between hexamers. The structures of all three hexamers are nearly identical, although a large displacement of the N-terminus of one B chain in both monoclinic structures results from coordination to a sodium ion which is located between symmetry-related hexamers. Other minor differences in structure arise from differences in packing in the monoclinic cell compared with the rhombohedral cell. Based upon the differences in conformation of the GluB13 side chains in T(6), T(3)R(f)(3) and R(6) hexamers, the deprotonation of these side chains appears to be associated with the T-->R conformational transition.
- Hauptman-Woodward Medical Research Institute, 73 High Street, Buffalo, NY 14203, USA. smith@hwi.buffalo.edu
Organizational Affiliation: