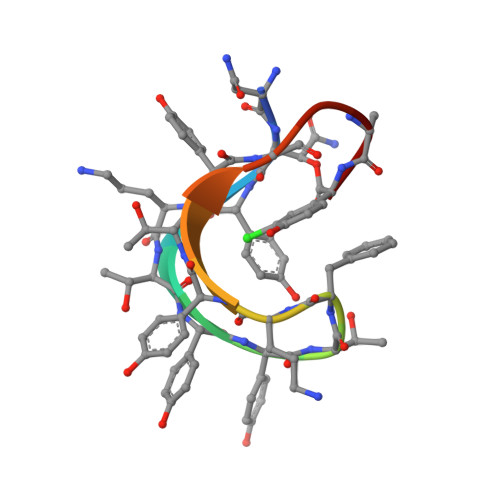
3D Structure of Ramoplanin: A Potent Inhibitor of Bacterial Cell Wall Synthesis.
Kurz, M., Guba, W.(1996) Biochemistry 35: 12570
- PubMed: 8823194
- DOI: https://doi.org/10.1021/bi961017q
- Primary Citation of Related Structures:
1DSR - PubMed Abstract:
The 3D structure of ramoplanin was studied by NMR spectroscopy in aqueous solution. A total of 320 interproton distances were determined from a NOESY spectrum and were used as restraints in distance geometry calculations. A structural refinement was carried out by molecular dynamics calculations in a solvent box. The structure of ramoplanin is characterized by two antiparallel beta-strands which are formed by the residues 2-7 and 10-14, respectively. The beta-strands are connected by six intramolecular hydrogen bonds and a reverse beta-turn which is formed by Thr8 and Phe9 (in positions i+1 and i+2, respectively). Residues 2 and 14 are connected by a loop consisting of Leu15, Ala16, Chp17, and the side chain of Asn2. Although residues 14-17 show the formation of a beta-turn, only the N-terminal end of the turn is directly connected to one of the beta-strands (Gly14), whereas the C-terminal end (Chp17) is linked via the side chain of Asn2. The 3D conformation of ramoplanin is also stabilized by a hydrophobic cluster of the aromatic sidechains of the residues 3, 9, and 17. This hydrophobic collapse leads to a U-shaped topology of the beta-shee: with the beta-turn at one end and the loop at the other end. The structure found for ramoplanin differs corsiderably from the published structure of ramoplanose which might be due to a smaller number of NOE distance restraints used in the previous study.
- Central Pharma Research, Frankfurt am Main, Germany.
Organizational Affiliation: