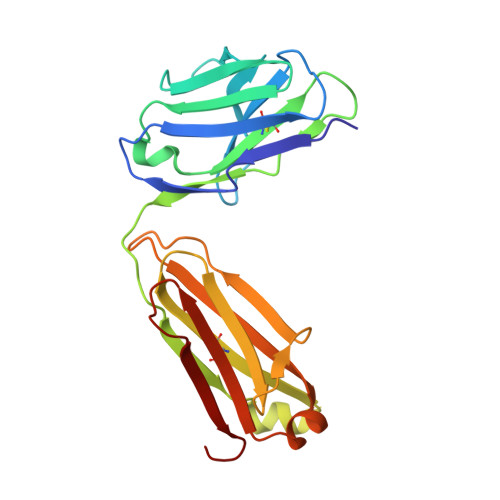
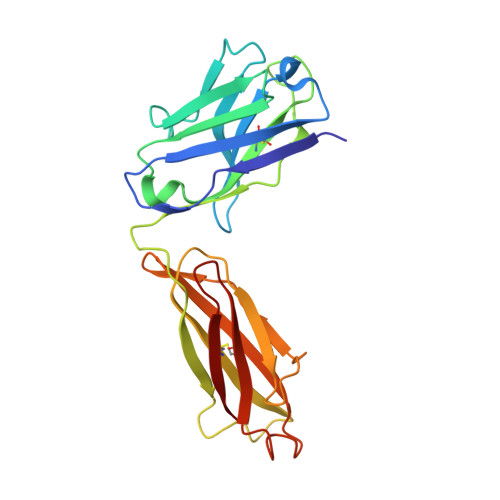
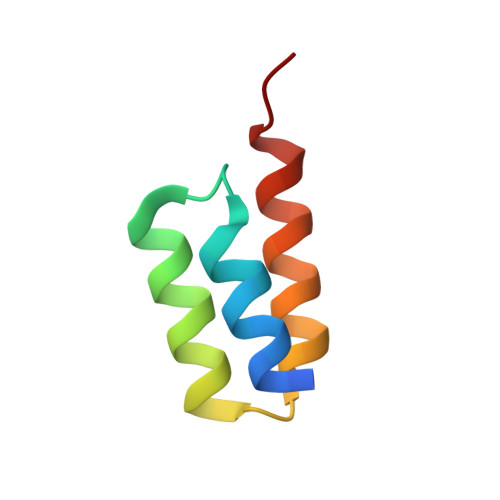
Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity.
Graille, M., Stura, E.A., Corper, A.L., Sutton, B.J., Taussig, M.J., Charbonnier, J.B., Silverman, G.J.(2000) Proc Natl Acad Sci U S A 97: 5399-5404
- PubMed: 10805799
- DOI: https://doi.org/10.1073/pnas.97.10.5399
- Primary Citation of Related Structures:
1DEE - PubMed Abstract:
Staphylococcus aureus produces a virulence factor, protein A (SpA), that contains five homologous Ig-binding domains. The interactions of SpA with the Fab region of membrane-anchored Igs can stimulate a large fraction of B cells, contributing to lymphocyte clonal selection. To understand the molecular basis for this activity, we have solved the crystal structure of the complex between domain D of SpA and the Fab fragment of a human IgM antibody to 2.7-A resolution. In the complex, helices II and III of domain D interact with the variable region of the Fab heavy chain (V(H)) through framework residues, without the involvement of the hypervariable regions implicated in antigen recognition. The contact residues are highly conserved in human V(H)3 antibodies but not in other families. The contact residues from domain D also are conserved among all SpA Ig-binding domains, suggesting that each could bind in a similar manner. Features of this interaction parallel those reported for staphylococcal enterotoxins that are superantigens for many T cells. The structural homology between Ig V(H) regions and the T-cell receptor V(beta) regions facilitates their comparison, and both types of interactions involve lymphocyte receptor surface remote from the antigen binding site. However, T-cell superantigens reportedly interact through hydrogen bonds with T-cell receptor V(beta) backbone atoms in a primary sequence-independent manner, whereas SpA relies on a sequence-restricted conformational binding with residue side chains, suggesting that this common bacterial pathogen has adopted distinct molecular recognition strategies for affecting large sets of B and T lymphocytes.
- Département d'Ingénierie et d'Etudes des Protéines (DIEP), Commissariat à l'Energie Atomique (CEA), C.E. Saclay, 91191 Gif-sur-Yvette Cedex, France.
Organizational Affiliation: