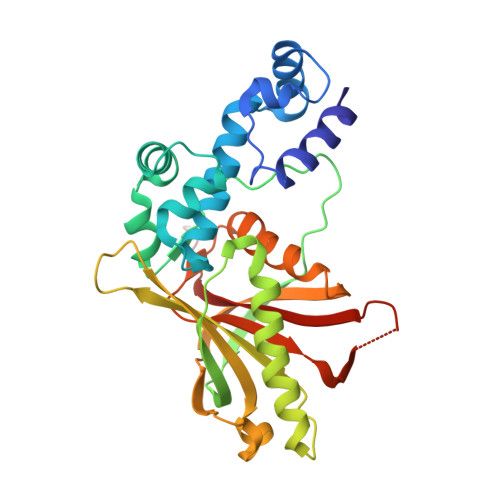
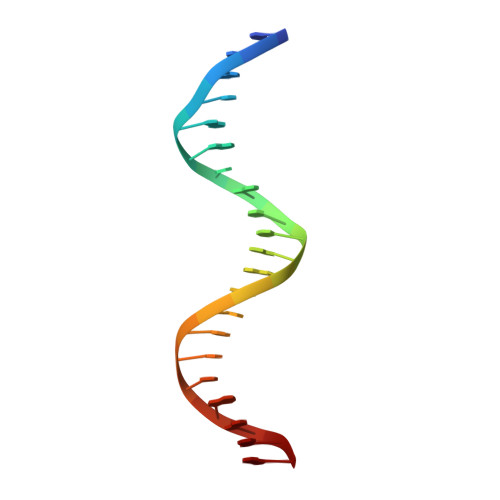
Crystal structure of the Escherichia coli Rob transcription factor in complex with DNA.
Kwon, H.J., Bennik, M.H., Demple, B., Ellenberger, T.(2000) Nat Struct Biol 7: 424-430
- PubMed: 10802742
- DOI: https://doi.org/10.1038/75213
- Primary Citation of Related Structures:
1D5Y - PubMed Abstract:
The Escherichia coli Rob protein is a transcription factor belonging to the AraC/XylS protein family that regulates genes involved in resistance to antibiotics, organic solvents and heavy metals. The genes encoding these proteins are activated by the homologous proteins MarA and SoxS, although the level of activation can vary for the different transcription factors. Here we report a 2.7 A crystal structure of Rob in complex with the micF promoter that reveals an unusual mode of binding to DNA. The Rob-DNA complex differs from the previously reported structure of MarA bound to the mar promoter, in that only one of Rob's dual helix-turn-helix (HTH) motifs engages the major groove of the binding site. Biochemical studies show that sequence specific interactions involving only one of Rob's HTH motifs are sufficient for high affinity binding to DNA. The two different modes of DNA binding seen in crystal structures of Rob and MarA also match the distinctive patterns of DNA protection by AraC at several sites within the pBAD promoter. These and other findings suggest that gene activation by AraC/XylS transcription factors might involve two alternative modes of binding to DNA in different promoter contexts.
- Graduate Program in Biophysics, Harvard University, Cambridge, MA 02138, USA.
Organizational Affiliation: