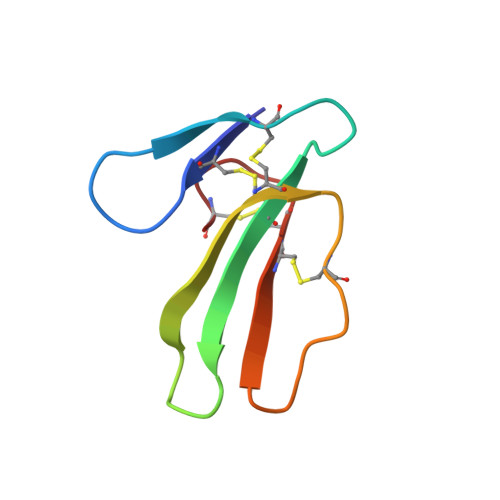
Refined three-dimensional solution structure of a snake cardiotoxin: analysis of the side-chain organization suggests the existence of a possible phospholipid binding site.
Gilquin, B., Roumestand, C., Zinn-Justin, S., Menez, A., Toma, F.(1993) Biopolymers 33: 1659-1675
- PubMed: 8241426
- DOI: https://doi.org/10.1002/bip.360331104
- Primary Citation of Related Structures:
1CXN, 1CXO - PubMed Abstract:
The solution structure of toxin gamma (60 residues, 4 disulfides) from Naja nigricollis was determined by proton nmr and molecular modeling with DIANA and X-PLOR. The structures were calculated using 489 distance and 81 dihedral angle constraints. The average atomic rms deviation between the nine refined structures and the average structure is 0.118 nm for the backbone atoms. Toxin gamma has an overall folding consisting of three loops stabilized by the four disulfides and forming a two- and a three-stranded beta-sheet (loop I and loops II, III, respectively). The same type of folding has been observed for two homologous cardiotoxins. The very close similarity of the solution structure of toxin gamma and the crystal structure of toxin VII4 includes details of the topological arrangement of numerous side chains. Among these are the conserved residues K12, K18, K35, and Y22, known to be critical for the cytolytic activity of toxin gamma. A cluster of hydrophobic side chains organized around Y22 is found on one side of the three-stranded beta-sheet and is spatially close to a group of three lysines (K12, K18, K35). The side chains of these lysines form a cationic site that can accommodate the binding of a phosphate ion as found in the crystal structure of toxin VII4. The hydrophobic cluster constitutes a possible binding site for the hydrophobic moiety of phospholipids. Together with the complementary cationic site, this hydrophobic surface can form a conserved site by which cardiotoxins bind to membrane phospholipids.
- Département d'Ingénierie et d'Etude des Protéines, CE-Saclay, Gif-sur-Yvette, France.
Organizational Affiliation: