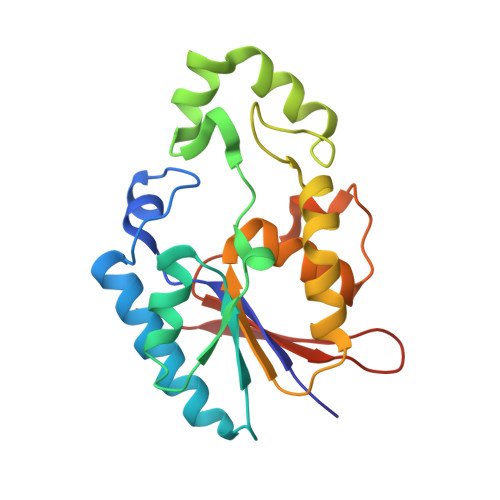
Reaction Mechanism of Fructose-2,6-bisphosphatase Suggested by the Crystal Structures of a pseudo-Michaelis complex and Metabolite Complexes
Lee, Y.-H., Olson, T.W., McClard, R.W., Witte, J.F., McFarlan, S.C., Banaszak, L.J., Levitt, D.G., Lange, A.J.To be published.