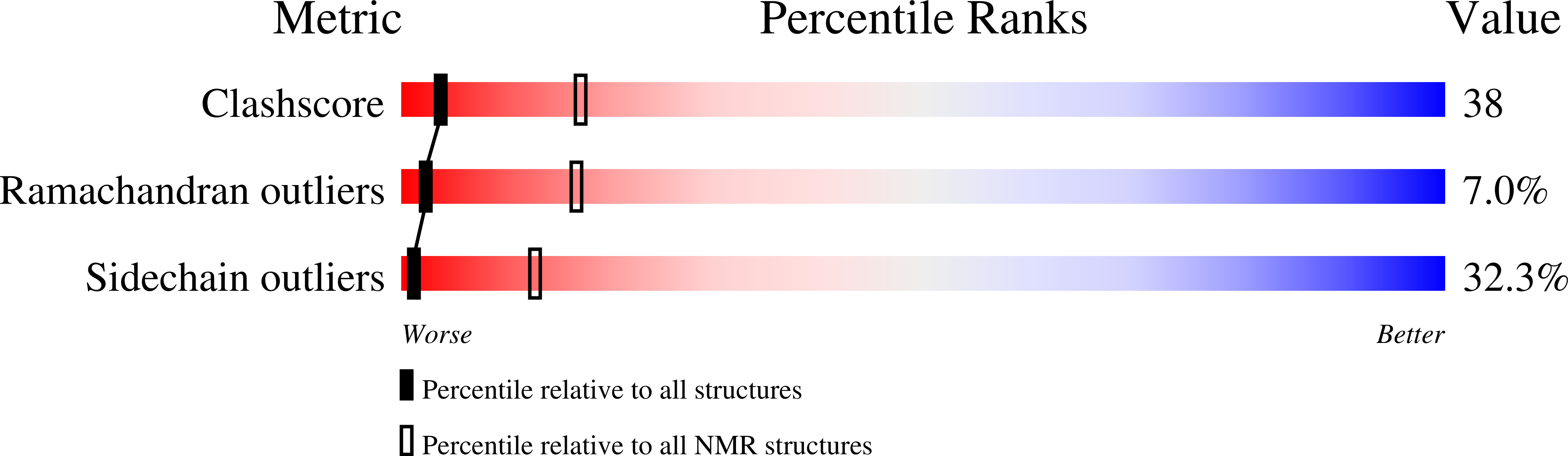Structural studies on the effects of the deletion in the red cell anion exchanger (band 3, AE1) associated with South East Asian ovalocytosis.
Chambers, E.J., Bloomberg, G.B., Ring, S.M., Tanner, M.J.(1999) J Mol Biology 285: 1289-1307
- PubMed: 9887277
- DOI: https://doi.org/10.1006/jmbi.1998.2392
- Primary Citation of Related Structures:
1BZK - PubMed Abstract:
We have carried out a solution-state NMR study of synthetic peptides patterned on the first membrane span of normal human band 3, and the same region of the mutant band 3 present in Southeast Asian ovalocytosis (SAO) which has a nine amino acid deletion. In 1:1 (v/v) chloroform/methanol, the 42 residue normal peptide (R389-K430) consisted of three helical regions. The slow solvent exchange of backbone amide protons revealed the helix from P403 to A416 was more stable than the "cytoplasmic" N-terminal helix from P391 to A400. These helices were separated by a sharp bend at P403, which is probably located at the boundary between the cytoplasmic domain and the first transmembrane span. The SAO deletion (A400-A408) removed the bend at P403, to leave a stable helix from P391 to A416 containing the residuum of the normal first transmembrane helix and with a hydrophobic turn replaced by a polar turn in the SAO peptide. Insertion of fragments of normal band 3 and band 3 SAO into microsomal membranes was investigated using a cell free translation system. A fragment composed of the cytoplasmic domain and the putative first membrane domain of normal band 3 (B3(1)) inserted stably into the membrane. However, the corresponding fragment of band 3 SAO [SAO(1)] did not integrate stably into membranes. Our results suggest that in SAO band 3, the region of the first membrane span of normal band 3 does not integrate properly into the membrane because it lacks a sufficiently long hydrophobic segment, and the deletion also disrupts a conserved structural subdomain at the membrane surface.
- Department of Biochemistry School of Medical Sciences, University of Bristol, Bristol, BS8 1TD, UK.
Organizational Affiliation: