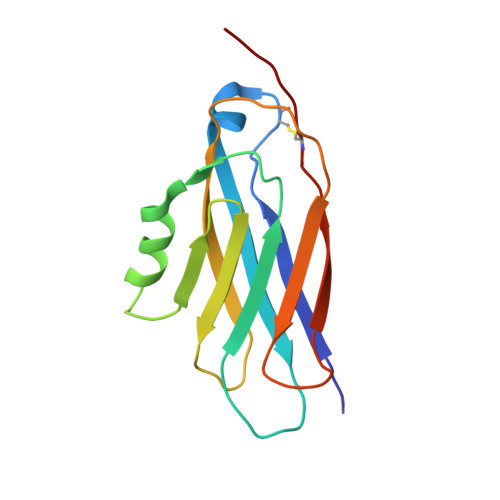
NMR solution structure of complement-like repeat CR8 from the low density lipoprotein receptor-related protein.
Huang, W., Dolmer, K., Gettins, P.G.(1999) J Biological Chem 274: 14130-14136
- PubMed: 10318830
- DOI: https://doi.org/10.1074/jbc.274.20.14130
- Primary Citation of Related Structures:
1BV8, 1CR8 - PubMed Abstract:
The low density lipoprotein receptor-related protein is a member of the low density lipoprotein receptor family and contains clusters of cysteine-rich complement-like repeats of about 42 residues that are present in all members of this family of receptors. These clusters are thought to be the principal binding sites for protein ligands. We have expressed one complement-like repeat, CR8, from the cluster in lipoprotein receptor-related protein that binds certain proteinase inhibitor-proteinase complexes and used three-dimensional NMR on the 13C/15N-labeled protein to determine the structure in solution of the calcium-bound form. The structure is very similar in overall fold to repeat 5 from the low density lipoprotein receptor (LB5), with backbone root mean square deviation of 1.5 A. The calcium-binding site also appears to be homologous, with four carboxyl and two backbone carbonyl ligands. However, differences in primary structure are such that equivalent surfaces that might represent the binding interfaces are very different from one another, indicating that different domains will have very different ligand specificities.
- Department of Biochemistry and Molecular Biology, College of Medicine, University of Illinois at Chicago, Chicago, Illinois 60612-4316, USA.
Organizational Affiliation: