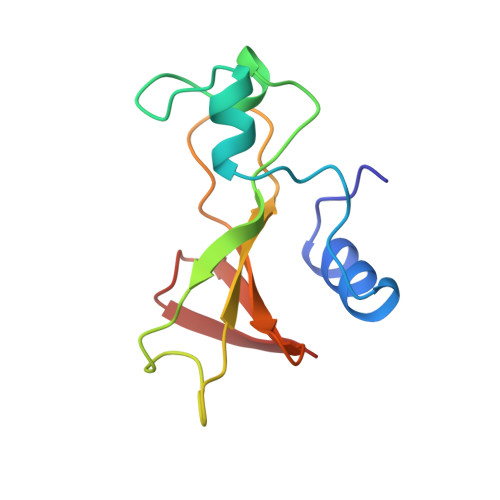
Three-dimensional structure of binase in solution.
Reibarkh, M.Y.a., Nolde, D.E., Vasilieva, L.I., Bocharov, E.V., Shulga, A.A., Kirpichnikov, M.P., Arseniev, A.S.(1998) FEBS Lett 431: 250-254
- PubMed: 9708913
- DOI: https://doi.org/10.1016/s0014-5793(98)00765-0
- Primary Citation of Related Structures:
1BUJ - PubMed Abstract:
We present the spatial structure of binase, a small extracellular ribonuclease, derived from 1H-NMR* data in aqueous solution. The total of 20 structures were obtained via torsion angle dynamics using DYANA program with experimental NOE and hydrogen bond distance constraints and phi and chi1 dihedral angle constraints. The final structures were energy minimised with ECEPP/2 potential in FANTOM program. Binase consists of three alpha-helices in N-terminal part (residues 6-16, 26-32 and 41-44), five-stranded antiparallel beta-sheet in C-terminal part (residues 51-55, 70-75, 86-90, 94-99 and 104-108) and two-stranded parallel beta-sheet (residues 22-24 and 49-51). Three loops (residues 36-39, 56-67 and 76-83), which play significant role in biological functioning of binase, are flexible in solution. The differences between binase and barnase spatial structures in solution explain the differences in thermostability of binase, barnase and their hybrids.
- Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, ul. Miklukho-Maklaya, Moscow.
Organizational Affiliation: