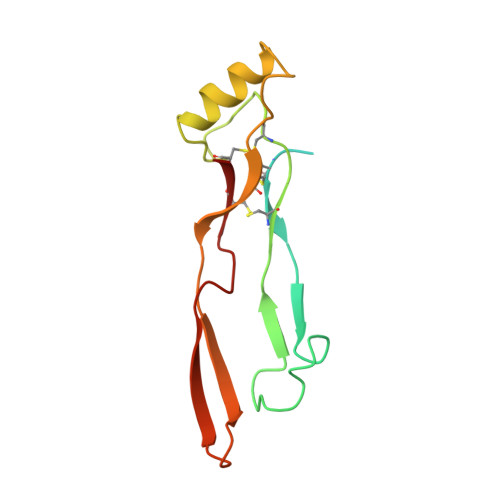
Three-dimensional structure of recombinant human osteogenic protein 1: structural paradigm for the transforming growth factor beta superfamily.
Griffith, D.L., Keck, P.C., Sampath, T.K., Rueger, D.C., Carlson, W.D.(1996) Proc Natl Acad Sci U S A 93: 878-883
- PubMed: 8570652
- DOI: https://doi.org/10.1073/pnas.93.2.878
- Primary Citation of Related Structures:
1BMP - PubMed Abstract:
We report the three-dimensional structure of osteogenic protein 1 (OP-1, also known as bone morphogenetic protein 7) to 2.8-A resolution. OP-1 is a member of the transforming growth factor beta (TGF-beta) superfamily of proteins and is able to induce new bone formation in vivo. Members of this superfamily share sequence similarity in their C-terminal regions and are implicated in embryonic development and adult tissue repair. Our crystal structure makes possible the structural comparison between two members of the TGF-beta superfamily. We find that although there is limited sequence identity between OP-1 and TGF-beta 2, they share a common polypeptide fold. These results establish a basis for proposing the OP-1/TGF-beta 2 fold as the primary structural motif for the TGF-beta superfamily as a whole. Detailed comparison of the OP-1 and TGF-beta 2 structures has revealed striking differences that provide insights into how these growth factors interact with their receptors.
- Rosenstiel Basic Medical Research Center, Brandeis University, Waltham, MA 02254, USA.
Organizational Affiliation: