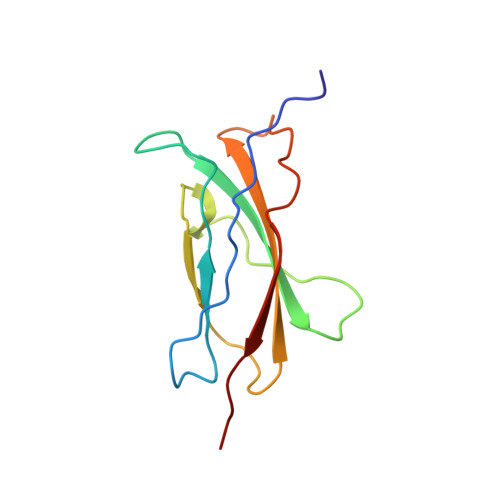
The signal transducer gp130: solution structure of the carboxy-terminal domain of the cytokine receptor homology region.
Kernebeck, T., Pflanz, S., Muller-Newen, G., Kurapkat, G., Scheek, R.M., Dijkstra, K., Heinrich, P.C., Wollmer, A., Grzesiek, S., Grotzinger, J.(1999) Protein Sci 8: 5-12
- PubMed: 10210178
- DOI: https://doi.org/10.1110/ps.8.1.5
- Primary Citation of Related Structures:
1BJ8 - PubMed Abstract:
The transmembrane glycoprotein gp130 is the common signal transducing receptor subunit of the interleukin-6-type cytokines. It is a member of the cytokine-receptor superfamily predicted to consist of six domains in its extracellular part. The second and third domain constitute the cytokine-binding module defined by a set of four conserved cysteines and a WSXWS motif, respectively. The three-dimensional structure of the carboxy-terminal domain of this region was determined by multidimensional NMR. The domain consists of seven beta-strands constituting a fibronectin type III-like topology. The structure reveals that the WSDWS motif of gp130 is part of an extended tryptophan/arginine zipper which modulates the conformation of the CD loop.
- Institut für Biochemie, Rheinisch-Westfälische Technische Hochschule Aachen, Germany.
Organizational Affiliation: