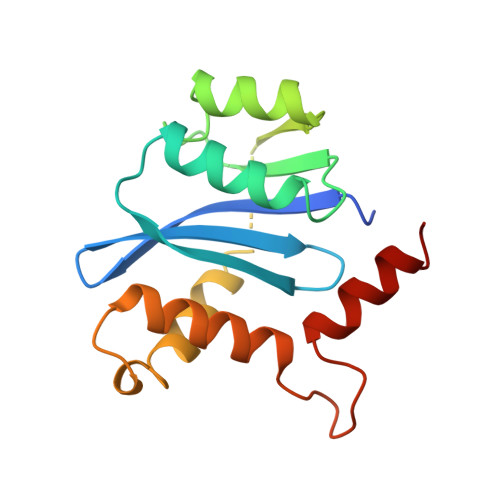
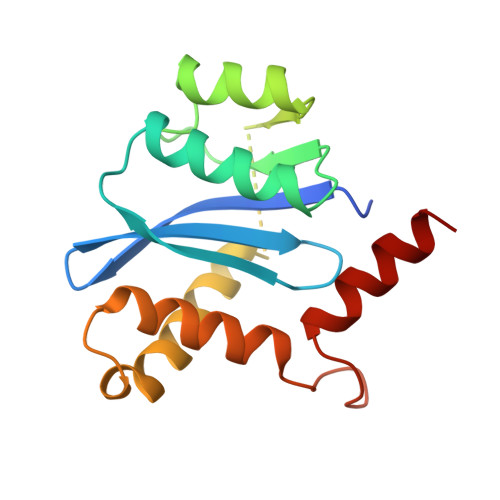
Crystal structures of the catalytic domain of HIV-1 integrase free and complexed with its metal cofactor: high level of similarity of the active site with other viral integrases.
Maignan, S., Guilloteau, J.P., Zhou-Liu, Q., Clement-Mella, C., Mikol, V.(1998) J Mol Biology 282: 359-368
- PubMed: 9735293
- DOI: https://doi.org/10.1006/jmbi.1998.2002
- Primary Citation of Related Structures:
1BHL, 1BI4, 1BL3 - PubMed Abstract:
Human immunodeficiency virus (HIV) integrase is the enzyme responsible for insertion of a DNA copy of the viral genome into host DNA, an essential step in the replication cycle of HIV. HIV-1 integrase comprises three functional and structural domains: an N-terminal zinc-binding domain, a catalytic core domain and a C-terminal DNA-binding domain. The catalytic core domain with the F185H mutation has been crystallized without sodium cacodylate in a new crystal form, free and complexed with the catalytic metal Mg2+. The structures have been determined and refined to about 2.2 A. Unlike the previously reported structures, the three active-site carboxylate residues (D,D-35-E motif) are well ordered and both aspartate residues delineate a proper metal-binding site. Comparison of the active binding site of this domain with that of other members from the polynucleotidyl transferases superfamily shows a high level of similarity, providing a confident template for the design of antiviral agents.
- Rhône-Poulenc Rorer, 13, Quai J. Guesde, Vitry/Seine, F-94403, France.
Organizational Affiliation: