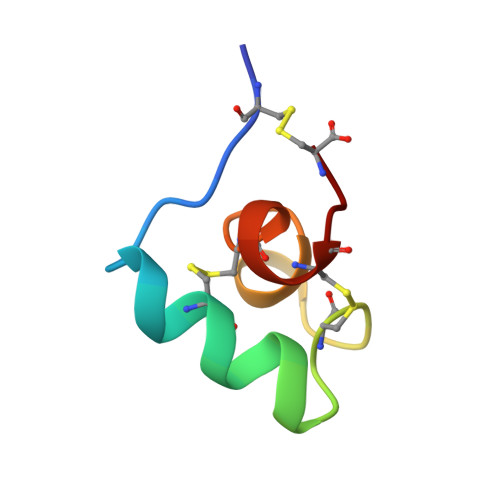
On the convergent evolution of animal toxins. Conservation of a diad of functional residues in potassium channel-blocking toxins with unrelated structures.
Dauplais, M., Lecoq, A., Song, J., Cotton, J., Jamin, N., Gilquin, B., Roumestand, C., Vita, C., de Medeiros, C.L., Rowan, E.G., Harvey, A.L., Menez, A.(1997) J Biological Chem 272: 4302-4309
- PubMed: 9020148
- DOI: https://doi.org/10.1074/jbc.272.7.4302
- Primary Citation of Related Structures:
1BGK - PubMed Abstract:
BgK is a K+ channel-blocking toxin from the sea anemone Bunodosoma granulifera. It is a 37-residue protein that adopts a novel fold, as determined by NMR and modeling. An alanine-scanning-based analysis revealed the functional importance of five residues, which include a critical lysine and an aromatic residue separated by 6.6 +/- 1.0 A. The same diad is found in the three known homologous toxins from sea anemones. More strikingly, a similar functional diad is present in all K+ channel-blocking toxins from scorpions, although these toxins adopt a distinct scaffold. Moreover, the functional diads of potassium channel-blocking toxins from sea anemone and scorpions superimpose in the three-dimensional structures. Therefore, toxins that have unrelated structures but similar functions possess conserved key functional residues, organized in an identical topology, suggesting a convergent functional evolution for these small proteins.
- Département d'Ingénierie et d'Etudes des Protéines, CEA, Saclay, 91191 Gif-sur-Yvette Cedex, France.
Organizational Affiliation: