Structural studies of a stable parallel-stranded DNA duplex incorporating isoguanine:cytosine and isocytosine:guanine basepairs by nuclear magnetic resonance spectroscopy.
Yang, X.L., Sugiyama, H., Ikeda, S., Saito, I., Wang, A.H.(1998) Biophys J 75: 1163-1171
- PubMed: 9726918
- DOI: https://doi.org/10.1016/S0006-3495(98)74035-4
- Primary Citation of Related Structures:
1BE5 - PubMed Abstract:
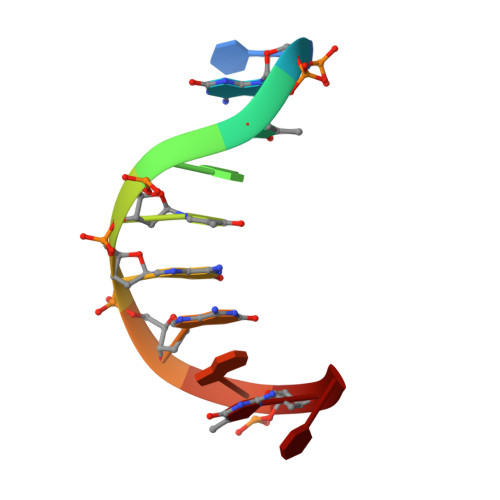
Isoguanine (2-hydroxyladenine) is a product of oxidative damage to DNA and has been shown to cause mutation. It is also a potent inducer of parallel-stranded DNA duplex structure. The structure of the parallel-stranded DNA duplex (PS-duplex) 5'-d(TiGiCAiCiGiGAiCT) + 5'-d(ACGTGCCTGA), containing the isoguanine (iG) and 5-methyl-isocytosine (iC) bases, has been determined by NMR refinement. All imino protons associated with the iG:C, G:iC, and A:T (except the two terminal A:T) basepairs are observed at 2 degrees C, consistent with the formation of a stable duplex suggested by the earlier Tm measurements [Sugiyama, H., S. Ikeda, and I. Saito. 1996. J. Am. Chem. Soc. 118:9994-9995]. All basepairs are in the reverse Watson-Crick configuration. The structural characteristics of the refined PS-duplex are different from those of B-DNA. The PS duplex has two grooves with similar width (7.0 A) and depth (7.7 A), in contrast to the two distinct grooves (major groove width 11.7 A, depth 8.5 A, and minor groove width 5.7 A, depth 7.5 A) of B-DNA. The resonances of the amino protons of iG and C are clearly resolved and observable, but those of the G and iC are very broad and difficult to observe. Several intercalators with different complexities, including ethidium, daunorubicin, and nogalamycin, have been used to probe the flexibility of the backbone of the (iG, iC)-containing PS-duplex. All of them produce drug-induced UV/vis spectra identical to their respective spectra when bound to B-DNA, suggesting that those drugs bind to the (iG, iC)-containing PS-duplex using similar intercalation processes. The results may be useful in the design of intercalator-conjugated oligonucleotides for antisense applications. The study presented in this paper augments our understanding of a growing number of parallel-stranded DNA structures, including the G-quartet, the i-motif, and the unusual homo basepaired parallel-stranded double helix. Their possible relevance is discussed.
- Department of Cell and Structural Biology, University of Illinois, Urbana-Champaign 61801, USA.
Organizational Affiliation: