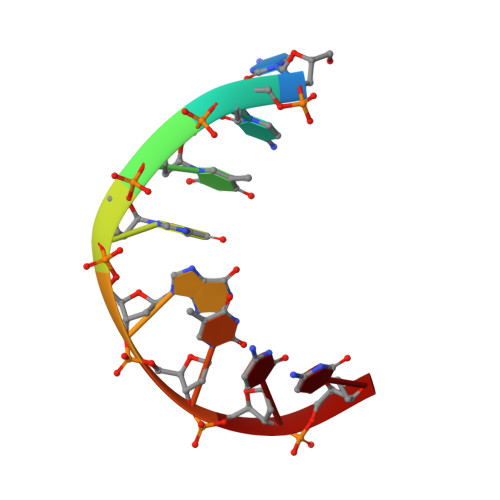
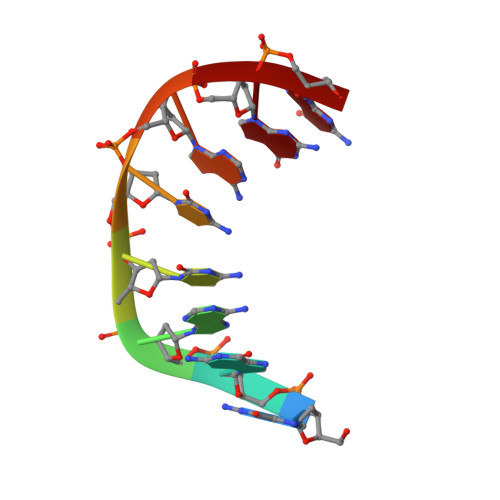
Structure and isomerization of an intrastrand cisplatin-cross-linked octamer DNA duplex by NMR analysis.
Yang, D., van Boom, S.S., Reedijk, J., van Boom, J.H., Wang, A.H.(1995) Biochemistry 34: 12912-12920
- PubMed: 7548048
- DOI: https://doi.org/10.1021/bi00039a054
- Primary Citation of Related Structures:
1AU5 - PubMed Abstract:
The anticancer platinum compound cis-Pt(NH3)2Cl2 (cisplatin) forms covalent cross-linked adducts with DNA, with the intrastrand didentate adduct between two adjacent guanines being the major product. The platinum atom is coordinated at the N7 positions of adjacent guanines. The duplex consisting of d(CCTG*G*TCC) and its complement d(GGACCAGG), where G*G* stands for the cisplatin cross-linked lesion site, has been analyzed by 1D- and 2D-NMR spectroscopy and its structure solved by the NOE-restrained refinement procedure with the aim to understand the structural distortion associated with the lesion. The refined duplex is unwound (approximately -21 degrees) and kinked (approximately 58 degrees) toward the major groove at the G*G* site, and the minor groove is significantly widened. The deoxyriboses of the G4* and G5* nucleotides are of the N-type (C3'-endo) and S-type (C2'-endo) conformations, respectively. The two guanine bases adopt the R-configuration (the alpha/beta angles being 112 degrees/290 degrees, respectively), such that the G5*H8 proton (upfield at 8.19 ppm) senses the ring current shielding effect of the G4* base (G4*H8 at 8.76 ppm). The G4*.C13 base pair is perturbed significantly, consistent with the lack of detection of its imino proton. The intrastrand Pt-G*pG* cross-link is metastable in the present DNA duplex. The molecule is slowly converted into a more stable interstrand didentate adduct (between G4 and G9) promoted by the presence of the nucleophilic chloride ion.(ABSTRACT TRUNCATED AT 250 WORDS)
- Biophysics Division, University of Illinois at Urbana-Champaign 61801, USA.
Organizational Affiliation: